Introduction Care Considerations supported by the Centers for Disease Control and Prevention for the management of Duchenne muscular dystrophy were published in 2010, but there has been limited study of implementation in the United States. Methods A questionnaire collecting information about standard care practices and perceived barriers was piloted by 9 clinic directors of facilities within the Muscular Dystrophy Surveillance, Tracking and Research network. Results Six clinic directors completed the questionnaire; 1 adult-only clinic was excluded. Over 80% adherence was found for 30 of 55 recommendations examined. Greatest variability was for initiation of corticosteroids, bone health monitoring, type of pulmonary function testing, and psychosocial management. Barriers included unclear guidelines, inadequate time and funding, family-specific barriers and lack of empirical support for some recommendations. Discussion This pilot study showed implementation of the 2010 Care Considerations, except for recommendations based largely on expert consensus. Complete adherence requires more studies and active promotion.
Introduction: Duchenne Muscular Dystrophy is a genetic disease that is caused by a deficiency of dystrophin protein. Both Duchenne Muscular Dystrophy patients and dystrophic mice suffer from intestinal dysfunction.
Methods: The present study arose from a chance observation of differences in fecal output of dystrophic vs. normal mice during 20minutes of forced continuous treadmill exercise. Here, we report on the effects of exercise on fecal output in two different dystrophic mutants and their normal background control strains. All fecal materials evacuated during exercise were counted, dried and weighed.
Results: Mice of both mutant dystrophic strains produced significantly more fecal material during the exercise bout than the relevant control strains.
Discussion: We propose that exercise-induced Colo-Rectal Activation Phenotype test could be used as a simple, highly sensitive, noninvasive biomarker to determine efficacy of dystrophin replacement therapies.
INTRODUCTION: Duchenne muscular dystrophy (DMD) is an X-linked genetic disorder that causes progressive skeletal and cardiac muscle weakness in boys. Cardiac dysfunction is a frequent cause of death in DMD. Glucocorticoids are the standard of care in DMD. The long-term use of oral glucocorticoids in DMD is complicated by poor bone health. Epidemiological studies suggest a biological link between the loss of bone mineral density (BMD) and cardiovascular disease, including coronary artery and cerebrovascular diseases. Whether an association between low BMD and cardiac dysfunction occurs in DMD boys has not yet been studied. The objective of this retrospective cohort study was to examine the relationship between BMD and cardiovascular health in DMD.
METHODS: Retrospective data analyses was performed from de-identified medical records from a tertiary academic medical center. Whole body BMD was measured using dual-energy xray absorptiometry (DEXA) scan and left ventricular ejection fraction (LVEF) was measured using echocardiogram. Linear regression was used to evaluate the relationship between BMD and LVEF.
RESULTS: Data was analyzed from a total of 32 boys with DMD. The mean age at which baseline BMD measurements was obtained of 11±3 (SD) years. The worst LVEF was measured at a mean of 23.7±21.8 (SD) months after the baseline BMD measurement. The final adjusted linear regression of the relationship between baseline BMD z-score and worst LVEF was not statistically significant (ß=0.41, p‑value=0.6455).
DISCUSSION: In this cohort of boys with DMD, BMD was not associated with LVEF dysfunction up to 79 months later. Future research with a longer longitudinal follow-up period is warranted to evaluate the relationship between BMD and cardiovascular disease in DMD.
The dystrophinopathies (Duchenne [DMD] and Becker muscular dystrophy) are progressive diseases that until recently had no specific treatments. New FDA pathways to drug approval in rare diseases have resulted in a dramatic increase in the number of treatment trials for DMD and recently, two approved drugs. Health insurance policies for DMD products have been constructed with limited input from neuromuscular specialists directly involved in patient care and without patient input. These policies often reflect a lack of understanding of the disease, clinical population or the treatment. To ensure that policy determinations reflect best clinical practice, we recommend insurers work with neuromuscular specialists with expertise in care for patients with dystrophinopathy, as well as patients and families, and prominent advocacy organizations, such as Parent Project Muscular Dystrophy, in developing policies.
Muscular dystrophy (MD) describes generalized progressive muscular weakness due to the wasting of muscle fibers. The progression of the disease is affected by known immunological and mechanical factors, and possibly other unknown mechanisms. These dynamics have begun to be elucidated in the last two decades. This article reviews mathematical models of MD and models that could be used to study molecular and cellular components implicated in MD progression. A biological background for these processes is also presented. Molecular effectors that contribute to MD include mitochondrial bioenergetics and genetic factors; both drive cellular metabolism, communication and signaling. These molecular events leave cells vulnerable to mechanical stress which can activate an immunological cascade that weakens cells and surrounding tissues. This review article lays the foundation for a systems biology approach to study MD progression.
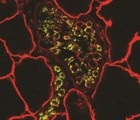
Histone acetyl transferases (HATs) and histone deacetylases (HDAC) control transcription during myogenesis. HDACs promote chromatin condensation, inhibiting gene transcription in muscle progenitor cells until myoblast differentiation is triggered and HDACs are released. HATs, namely CBP/p300, activate myogenic regulatory and elongation factors promoting myogenesis. HDAC inhibitors are known to improve regeneration in dystrophic muscles through follistatin upregulation. However, the potential of directly modulating HATs remains unexplored. We tested this possibility in a well-known zebrafish model of Duchenne muscular dystrophy. Interestingly, CBP/p300 transcripts were found downregulated in the absence of Dystrophin. While investigating CBP rescuing potential we observed that dystrophin-null embryos overexpressing CBP actually never show significant muscle damage, even before a first regeneration cycle could occur. We found that the pan-HDAC inhibitor trichostatin A (TSA) also prevents early muscle damage, however the single HAT CBP is as efficient even in low doses. The HAT domain of CBP is required for its full rescuing ability. Importantly, both CBP and TSA prevent early muscle damage without restoring endogenous CBP/p300 neither increasing follistatin transcripts. This suggests a new mechanism of action of epigenetic regulators protecting dystrophin-null muscle fibres from detaching, independent from the known improvement of regeneration upon damage of HDACs inhibitors. This study builds supporting evidence that epigenetic modulators may play a role in determining the severity of muscle dystrophy, controlling the ability to resist muscle damage. Determining the mode of action leading to muscle protection can potentially lead to new treatment options for muscular dystrophies in the future.
INTRODUCTION: Both genetic and infectious diseases can result in skeletal muscle degeneration, inflammation, pain, and/or weakness. Duchenne muscular dystrophy (DMD) is the most common congenital muscle disease. DMD causes progressive muscle wasting due to mutations in Dystrophin. Influenza A and B viruses are frequently associated with muscle complications, especially in children. Infections activate an immune response and immunosuppressant drugs reduce DMD symptoms. These data suggest that the immune system may contribute to muscle pathology. However, roles of the immune response in DMD and Influenza muscle complications are not well understood. Zebrafish with dmd mutations are a well-characterized model in which to study the molecular and cellular mechanisms of DMD pathology. We recently showed that zebrafish can be infected by human Influenza A virus (IAV). Thus, the zebrafish is a powerful system with which to ask questions about the etiology and mechanisms of muscle damage due to genetic and/or infectious diseases.
METHODS: We infected zebrafish with IAV and assayed muscle tissue structure, sarcolemma integrity, cell-extracellular matrix (ECM) attachment, and molecular and cellular markers of inflammation in response to IAV infection alone or in the context of DMD.
RESULTS: We find that IAV-infected zebrafish display mild muscle degeneration with sarcolemma damage and compromised ECM adhesion. An innate immune response is elicited in muscle in IAV-infected zebrafish: NFkB signaling is activated, pro-inflammatory cytokine expression is upregulated, and neutrophils localize to sites of muscle damage. IAV-infected dmd mutants display more severe muscle damage than would be expected from an additive effect of dmd mutation and IAV infection, suggesting that muscle damage caused by Dystrophin-deficiency and IAV infection is synergistic.
DISCUSSION: These data demonstrate the importance of preventing IAV infections in individuals with genetic muscle diseases. Elucidating the mechanisms of immune-mediated muscle damage will not only apply to DMD and IAV, but also to other conditions where the immune system, inflammation, and muscle tissue are known to be affected, such as autoimmune diseases, cancer, and aging.
Introduction: Duchenne Muscular Dystrophy (DMD) is a debilitating muscle wasting disorder with no cure. Safer supplements and therapies are needed to improve the severity of symptoms, as severe side effects are associated with the only effective treatment, corticosteroids. The amino acid taurine has shown promise in ameliorating dystrophic symptoms in mdx mice, an animal model of DMD, however little work is in 21-28 (d)ay animals, the period of natural peak damage.
Methods: This study compares the effect of prenatal taurine supplementation on tibialis anterior (TA) in situ contractile function, histopathological characteristics and the abundance of Ca2+-handling as well as pathologically relevant proteins in non-exercised mdx mice at 28 and 70 d.
Results: Supplementation elevated TA taurine content by 25% (p<0.05), ameliorated in situ specific force by 60% (p<0.05) and improved histological characteristics in 28 d mdx mice; however no benefit was seen in 70 d mice, where background pathology was initially stable. Age specific effects in SERCA1, calsequestrin 1 (CSQ1), CSQ2, utrophin and myogenin protein abundances were seen between both 28 and 70 d mdx and mdx taurine-supplemented mice.
Discussion: Considering these findings and that taurine is a relatively cost effective, readily accessible and side effect free dietary supplement, we propose further investigation into taurine supplementation during pregnancy in a protective capacity, reminiscent of folate in the prevention of spinal bifida.
To facilitate gene and cell therapy experiments, we created severely immune-deficient mouse models of Duchenne muscular dystrophy (DMD), limb girdle muscular dystrophy 2B (LGMD2B), and limb girdle muscular dystrophy 2D (LGMD2D) by crossing mdx4Cv, Bl/AJ, and Sgca-null mice with NRG immune-deficient mice. The resulting mdx4Cv/NRG, BlAJ/NRG, and Sgca/NRG mice demonstrated the presence of the appropriate mutant alleles at Dmd, Dysf, Sgca, Rag1, and Il2rγ by genotyping PCR. Absence of dystrophin, dysferlin, or α-sarcoglycan protein was confirmed by western blot and immunohistochemistry. We performed centronucleation, Evans blue dye, hydroxyproline, and treadmill assays on the disease model mice versus NRG controls to evaluate muscle histology and function. These studies demonstrated that the mdx4Cv/NRG and Sgca/NRG mice showed significant deficits in muscle structure and function in all the assays and were similar to each other. By contrast, the phenotype of the BlAJ/NRG mice was milder in each case. The results we observed parallel the phenotypes seen in patients with the corresponding disorders. These novel immune-deficient mouse models of DMD, LGMD2B, and LGMD2D will be useful for long-term gene and cell therapy studies involving transfer of foreign genes and cells.
Duchenne muscular dystrophy (DMD or Duchenne) is a progressive, life-limiting muscle-wasting disease that requires comprehensive, multidisciplinary care. This care, at minimum, should include neuromuscular, respiratory, cardiac, orthopedic, endocrine and rehabilitative interventions that address both the primary and secondary manifestations of the disease. The care needs of patients evolve over the cdourse of the disease and as they transition from childhood into young adulthood. In the past two decades, life expectancy has increased significantly by the use of corticosteroids and enhanced clinical management. Nevertheless, each year, patients with Duchenne muscular dystrophy are admitted to emergency departments and intensive care units where medical expertise thrives, but where expertise in rare diseases, such as Duchenne, may not. Emergency care for patients with Duchenne can be as complex as the disease process itself. While any illness or injury may occur in a person with Duchenne, some acute scenarios are much more common in the context of the disease. Making decisions about the clinical care of a person with Duchenne who presents with an acute illness can be quite difficult — in part, because of the extensive use of corticosteroids, which can lead to adrenal suppression. The life of a person with Duchenne needing emergency care may therefore depend upon the ability of the clinician on duty in the emergency department to recognize and mitigate adrenal suppression resulting from corticosteroid dependence. With this in mind, and drawing from expertise and experience with other steroid-dependent diseases, the ‘PJ Nicholoff Steroid Protocol’ was developed. The purpose of this protocol is to provide clinicians information regarding the safe management of corticosteroid during emergency situations in patients who may have accompanying adrenal suppression. The protocol explains how to recognize the signs and symptoms of acute adrenal crisis, how to prevent it with supplemental stress doses of corticosteroids, and how to taper doses after emergency care in order to prevent corticosteroid withdrawal.