Abstract
Background:
21 days has been regarded as the appropriate quarantine period for holding individuals potentially exposed to Ebola Virus (EV) to reduce risk of contagion. There does not appear to be a systematic discussion of the basis for this period.
Methods:
The prior estimates for incubation time to EV were examined, along with data on the first 9 months of the current outbreak. These provided estimates of the distribution of incubation times.
Results:
A 21 day period for quarantine may result in the release of individuals with a 0.2 – 12% risk of release prior to full opportunity for the incubation to proceed. It is suggested that a detailed cost-benefit assessment, including considering full transmission risks, needs to occur in order to determine the appropriate quarantine period for potentially exposed individuals.
Funding Statement
No external funding was received for this work. The author has declared that no competing interests exist.Introduction
For contagious diseases, to reduce the spread, it is necessary and desirable to quarantine each individual who might have been exposed for a sufficient time for either infection to occur or until it can be assured that there is not likely to be infection (and hence spread of contagion). According to the Centers for Disease Control and Prevention1:
“When someone has been exposed to a contagious disease and it is not yet known if they have caught it, they may be quarantined or separated from others who have not been exposed to the disease. For example, they may be asked to remain at home to prevent further potential spread of the illness. They also receive special care and observation for any early signs of the illness.”
Parenthetically, if an individual manifests progress into a contagious state, subsequent confinement may occur until the contagiousness ends. The question then arises how long should the quarantine of an individual occur to provide assurance that progress to an infectious state would not result.
Sartwell2 conducted one of the first systematic studies on the incubation time for human pathogens and found that a broad spectrum of agents had incubation time distributions that could be modeled as lognormal (although alternative distributional forms were not tested). Leclerc et al. 3 examined the incubation time distribution of a variety of plant pathogens and observed that they could be fit (depending on the pathogen and the plant age) by either the gamma, lognormal, or Weibull distributions. All of these three are skewed right. Williams looked at the theoretical incubation time distribution for pathogens conforming to a stochastic in vivo birth-death process and found that they could also be characterized by a skewed distribution4,5.
In general none of the often used incubation time distributions have a maximum upper limit. In other words
Therefore there is no quarantine time that will provide absolute assurance of no residual risk from contagion. Nishiura6 pointed out the importance of examining the upper tail of the incubation time distribution when assessing the quarantine period following exposure to smallpox. This was also discussed in the context of the SARS coronavirus outbreak7 . Both of these previous authors noted the importance of the distributional form in assessing the upper tail probability, and the influence that data truncation may have on such estimates.
To make use of this approach, an acceptable residual risk needs to be set. To do this, one needs to balance out the costs and benefits of quarantine and risk reduction. For contagious diseases this will require coupling the risk of premature release from quarantine to a disease transmission model such as Legrand et al. 8. This can schematically be shown as in Figure 1. If the costs for enforcing quarantine up to a given time (post exposure) and if the costs associated with releasing individuals at a given time post exposure (with concomitant probability of eventually becoming contagious) can both be estimated, then the optimal quarantine time should be at the intersection of the curves given that both are plotted using the same y-axis. Clearly for pathogens that have a high degree of transmissibility and/or a high degree of severity, the quarantine time should be greater than for agents with lower transmissibility and/or severity. The purpose of this paper is not to estimate where the balancing point should be.
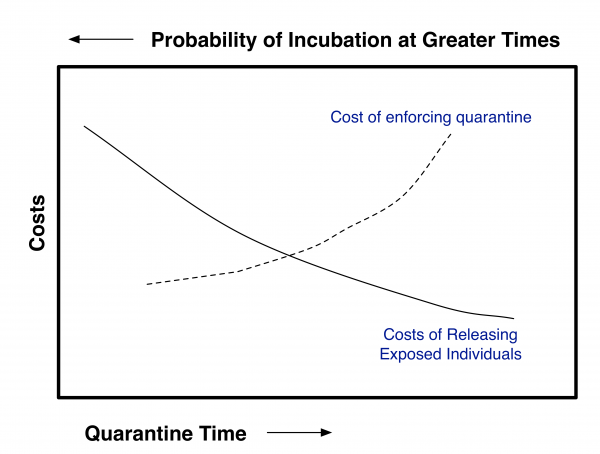
Fig. 1: Schematic of Tradeoff Analysis Needed to Determine Optimum Quarantine Time
The current operative guidance on quarantine periods for Ebola (Zaire) virus is 21 days, based on WHO assessment that the incubation period is 2–21 days 9. A current review of previous outbreaks cites the same range 10. The precise origin of this assessment is unclear, however it is possibly based on the study of the either the 1976 Zaire outbreak11 or 2000 Uganda outbreak12 both of which reported (without detailed analysis) a maximum observed incubation time of 21 days.
This contribution will outline what is known about the incubation time distribution for Ebola (Zaire), and what the potential upper tail percentiles might be. With additional information as outlined above, a formal decision analytic approach to deducing the optimal quarantine time may be devised. In view of the current concerns for the Ebola outbreak in West Africa, illustrations using this organism will be presented.
Prior Knowledge on Ebola Incubation Time Distributions
Breman et al.11 did a detailed analysis of the 1976 Zaire outbreak by examining lags between a person-to-person case and the time at which presumed exposure occurred. In their analysis of 109 such cases, they report a mean of 6.3 days and a range of 1 to 21 days, with no distributional fit. However they do report all of the individual observed times.
Chowell et al. 13 analyzed the 1995 Congo and 2000 Uganda outbreaks using an SEIR 14 model. For the S → E → I transition the equations become:
The term kE reflects the conversion rate of exposed to infected. In this model, which has been very frequently used for infectious disease transmission modeling, the implied incubation time distribution is an exponential one with a mean incubation time of 1/k. This can be seen by taking β to zero and observing that the solution to dE/dt is exponential. β is the transmission rate and γ is the rate of exit from the infectious state. The transmission rate may change during the course of an outbreak — e.g., with increasing control mechanisms it may decrease.
Using this analysis Chowell et al. 13 found the mean and standard deviation of the incubation period for the two outbreaks to be:
Outbreak
1/k (days) best estimate
standard deviation of the estimate
Congo 1995 (315 Cases)
5.30
0.23
Uganda 2000 (425 Cases)
3.35
0.49
Lekone and Finkenstadt15 analyzed the Congo 1995 outbreak (291 cases) using a Bayesian approach and a stochastic SEIR model. Their analysis yielded a larger value for the mean incubation time — 10.11 days using an informative prior. Their approach used an SEIR formulation and thus also an implied exponential distribution.
Eichner et al.16 analyzed the 1995 Congo outbreak assuming a lognormal distribution for incubation time (173 cases examined). No assessment of goodness of fit to this distribution was made. They estimated the mean incubation time of 12.7 days with a standard deviation of 4.31 days.
The WHO Response Team17 has just published an incubation time distribution based on the first 9 months of the West Africa outbreak (total of 4010 confirmed and probable cases with usable data). They reported a mean incubation period of 11.4 days with an upper 95th percentile of 21 days — and they were able to fit the data to a gamma distribution.
Results and Conclusions
Figure 2 plots the complementary cumulative distributions from the studies noted above.
Eichner is lognormal. Breman is the empirical distribution. The W. Africa distribution is gamma. The other distributions are exponential. For reference, a vertical line is shown at 21 days.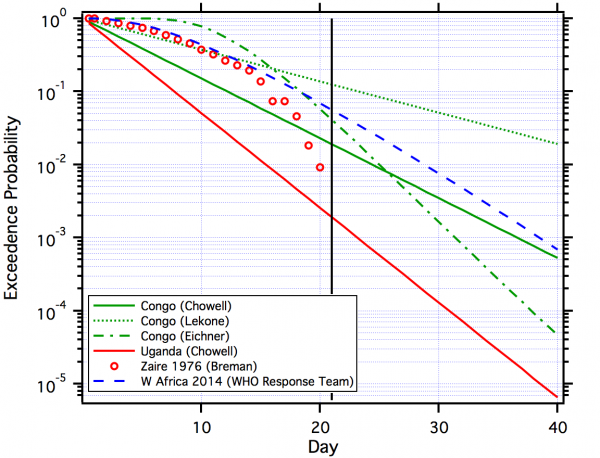
Fig. 2: Comparison of Estimated Distributions for EBOV.
Several aspects are clear:
In discussing the quarantine period for smallpox Nishiura18 discussed the use of the upper 95th percentile of the incubation time distribution , although this may vary with the presence of asymptomatic cases and R0 13. If the estimated incubation time distributions for the Congo outbreak (Figure 2) are used, this would suggest a quarantine time of as high as 31 days. But as noted above, the set point (95th, 99th, etc.) for a decision making needs to be based on balancing costs and benefits.
It should also be noted that the functional form used for the incubation time distribution will have an effect on the estimated upper tail probabilities (as well as the mean itself); this was illustrated in the case of SARS 19. This is analogous to the problem of differentiating between distributions in risk assessment20. However other candidate skewed distributions should be tested against available data sets.
The focus of the above discussion has been assessing the best estimates of the incubation time distribution in order to develop a rational process for Ebola quarantine estimation. However any estimated distribution has uncertainty, e.g. as shown in Table 1. Therefore the confidence bands of the distribution (e.g., confidence limits to the upper 95th percentile of the incubation time distribution) should be estimated and used in setting a quarantine time.
While the 21 day quarantine value currently used may have arose from reasonable interpretation of early outbreak data, this work suggests a reconsideration is in order and that 21 days may not be sufficiently protective to public health. Further, outbreaks such as the current West Africa EBOV are presenting an opportunity for careful collection of data sufficient to revise and update (perhaps in an adaptive fashion) such recommendations. It may be that incubation time itself is a function of intensity and nature of contact21, which may also need to be considered. The estimate of appropriate incubation time would need to explicitly consider the costs and benefits involved in various alternatives, which would incorporate explicit computations from transmission modeling.
Acknowledgements
I thank Prof. Dr. Martin Eichner for clarifying points in his 2011 paper and Prof. Christl Donnelly for supplying the parameters of the best fitting gamma distribution for the 2014 ongoing outbreak. I thank the reviewers for significant comments improving the strength of the argument.References
- CDC Emergency Risk Communication Branch (ERCB). Understand quarantine and isolation: Questions & answers, 2014.
- SARTWELL PE. The distribution of incubation periods of infectious disease. Am J Hyg. 1950 May;51(3):310-8. PubMed PMID:15413610.
- Leclerc M, Doré T, Gilligan CA, Lucas P, Filipe JA. Estimating the delay between host infection and disease (incubation period) and assessing its significance to the epidemiology of plant diseases. PLoS One. 2014;9(1):e86568. PubMed PMID:24466153.
- Williams, T., The Basic Birth-Death Model for Microbial Infections. Journal of the Royal Statistical Society Part B, 1965. 27: p. 338-360.
- Williams T. The distribution of response times in a birth-death process. Biometrika. 1965 Dec;52(3):581-5. PubMed PMID:5858972.
- Nishiura H. Determination of the appropriate quarantine period following smallpox exposure: an objective approach using the incubation period distribution. Int J Hyg Environ Health. 2009 Jan;212(1):97-104. PubMed PMID:18178524.
- Farewell VT, Herzberg AM, James KW, Ho LM, Leung GM. SARS incubation and quarantine times: when is an exposed individual known to be disease free? Stat Med. 2005 Nov 30;24(22):3431-45. PubMed PMID:16237660.
- Legrand J, Grais RF, Boelle PY, Valleron AJ, Flahault A. Understanding the dynamics of Ebola epidemics. Epidemiol Infect. 2007 May;135(4):610-21. PubMed PMID:16999875.
- Briand S, Bertherat E, Cox P, Formenty P, Kieny MP, Myhre JK, Roth C, Shindo N, Dye C. The international Ebola emergency. N Engl J Med. 2014 Sep 25;371(13):1180-3. PubMed PMID:25140855.
- Del Rio C, Mehta AK, Lyon Iii GM, Guarner J. Ebola Hemorrhagic Fever in 2014: The Tale of an Evolving Epidemic. Ann Intern Med. 2014 Aug 19. PubMed PMID:25133433.
- JG Breman, P Piot, KM Johnson, MK White, M Mbuyi, P Sureau, DL Heymann, S Van Nieuwen- hove, JB McCormick, and JP Ruppol. The epidemiology of Ebola hemorrhagic fever in Zaire, 1976, pages 85–97. Elsevier/North Holand Biomedical Press, Amsterdam, 1978.
- Okware SI, Omaswa FG, Zaramba S, Opio A, Lutwama JJ, Kamugisha J, Rwaguma EB, Kagwa P, Lamunu M. An outbreak of Ebola in Uganda. Trop Med Int Health. 2002 Dec;7(12):1068-75. PubMed PMID:12460399.
- Chowell G, Hengartner NW, Castillo-Chavez C, Fenimore PW, Hyman JM. The basic reproductive number of Ebola and the effects of public health measures: the cases of Congo and Uganda. J Theor Biol. 2004 Jul 7;229(1):119-26. PubMed PMID:15178190.
- R.M. Anderson and Robert M. May. Infectious Diseases in Humans: Dynamics and Control. Oxford University Press, Oxford, 1991.
- Lekone PE, Finkenstädt BF. Statistical inference in a stochastic epidemic SEIR model with control intervention: Ebola as a case study. Biometrics. 2006 Dec;62(4):1170-7. PubMed PMID:17156292.
- Eichner M, Dowell SF, Firese N. Incubation period of ebola hemorrhagic virus subtype zaire. Osong Public Health Res Perspect. 2011 Jun;2(1):3-7. PubMed PMID:24159443.
- Ebola Virus Disease in West Africa - The First 9 Months of the Epidemic and Forward Projections. N Engl J Med. 2014 Sep 22. PubMed PMID:25244186.
- Nishiura H, Wilson N, Baker MG. Quarantine for pandemic influenza control at the borders of small island nations. BMC Infect Dis. 2009 Mar 11;9:27. PubMed PMID:19284571.
- Cowling BJ, Muller MP, Wong IO, Ho LM, Louie M, McGeer A, Leung GM. Alternative methods of estimating an incubation distribution: examples from severe acute respiratory syndrome. Epidemiology. 2007 Mar;18(2):253-9. PubMed PMID:17235210.
- C. N. Haas. Importance of distributional form in characterizing inputs to Monte Carlo risk assessment. Risk Analysis, 17(1):107–113, 1997.
- Williams T, Meynell GG. Time-dependence and count-dependence in microbial infection. Nature. 1967 Apr 29;214(5087):473-5. PubMed PMID:4291777.

Leave a Comment
You must be logged in to post a comment.