Abstract
Development of six large nodules of solid tissue after bilateral human fetal striatal transplantation in four Huntington’s disease patients has raised concern about the safety of this experimental therapy in our setting. We investigated by serial MRI-based volumetric analysis the growth behaviour of such grafts. After 33-73 months from transplantation the size of five grafts was stable and one graft showed a mild decrease in size. Signs neither of intracranial hypertension nor of adjuctive focal neurological deficit have ever been observed. This supports long-term safety of the grafting procedure at our Institution.
Funding Statement
This study was supported by the University of Florence and Azienda Ospedaliero-Universitaria Careggi, Florence, and by grants from Ente Cassa di Risparmio di Firenze and MIUR (PRIN 2008, research program N 2008XN9KLA).Introduction
Huntington’s disease is an incurable neurodegenerative disorder for which human fetal striatal transplantation is being explored as an experimental approach with uncertain long-term results.1,2,3 Recently, Paganini et al.4 reported that fetal striatal grafting slowed the progression of motor and cognitive decline in patients participating in the Florence transplantation program. This renewed the interest in such a therapeutic strategy in Huntington’s disease.5 This notwithstanding, several challenges remain to be faced, not least the long-term fate of the graft. In most Huntington’s disease transplantation studies the grafted material showed some form of development, but the way and the extent of the growth greatly varied both among and within trials.6 In four patients of the Florentine series, six of eight grafts resulted in the growth of large tissue nodules overcoming the size of native striatum.7 This behaviour was deemed as “overgrowth” and raised much concern about the safety of our transplantation procedure.8 In particular, it was anticipated a possible adverse effect of the “marked cellular overgrowth” on the clinical status of the patients with particular emphasis on the risk of development of fatal mass lesions and even the ultimate necessity of surgical removal of graft tissue.8 More recently, Cisbani and Cicchetti6 re-launched worries on the outcome of our large-sized grafts. We report here the demonstration by magnetic resonance imaging volumetric analysis that the growth of these grafts stopped within the fourth quarter after transplantation and remained stable in the long term.
Methods
Ethics Statement. The present study has been conducted according to the Declaration of Helsinki principles. The patients provided written informed consent. The transplantation program was authorized by the Italian National Health Institute, National Transplantation Centre (upon approval by Health Ministry, Consiglio Superiore di Sanità, Sessione XLV, Sezione II, 7/21 and 9/22/2005, and acceptance by the National Bioethics Committee).
Human fetal striatal transplantation at our Institution implies bilateral (two months apart) caudate-putaminal stereotactic-robotic (NeuroMate Robotics System, Schaerermayfield, France) grafting of a tissue suspension obtained from both whole ganglionic eminencies of a single legally aborted human fetus (9–12 weeks gestation).7 All magnetic resonance imaging examinations of these four patients (n=20, 3–7 each) from baseline (9–12 months after grafting, Figure 1a) to those obtained at the last available follow-up (range 33-73 months after grafting) were considered here. Examinations were carried out under mild sedation on the same 1.5T unit and the protocol included T2-weighted and contrast-enhanced high-resolution 3D T1-weighted images. Using the Striker navigation system workstation (Howmedica Leibinger, Freiburg, Germany) a neuroradiologist manually contoured the entire grafts in axial 3D T1-weighted MPRAGE (1 mm thick, 256×256 matrix) images with real time control of the segmentation on images reformatted on the sagittal and coronal planes available on complementary viewing windows. The volume of the grafts was automatically calculated. At least two measurements per graft per scan (total 81, mean 2.9) were taken at a 2-week interval by the same operator.
Results
The coefficient of variations (CV = standard deviation/mean) were all below 10%. The largest graft was by far n. 4 with a mean volume of 42.0±1.8 mL and 43.0±1.9 mL at 10 and 18 months after surgery, respectively, then decreased by 20% and remained unchanged at 45-54 months. The volumes of the other five grafts at baseline ranged between 5.3 and 15.9 mL. Their size remained stable, namely the regression lines of volumes over time had slopes not significantly different from 0. Mean volume measurements over time are displayed in Figure 1b.
Figure 1: (a) Contrast-enhanced T1-weighted (grafts n. 1, 3 and 4) and T2-weighted (grafts n. 2, 5 and 6) magnetic resonance images obtained 9-12 months after human fetal striatal transplantation in four Huntington’s disease patients in which grafts attained a large size. (b) Mean of repeated volume measurements for each of the six grafts at each patient-specific magnetic resonance examination follow-up time are plotted. A series of linear mixed models with graft-specific random intercepts were fitted. Linearity tests satisfied the simple regression model for grafts n. 1, 2, 3, 5, and 6. The estimated slopes were, respectively, -0.004, 0.026, 0.010, 0.002, and 0.001, all not significantly different from 0. In the case of graft n. 4, the appropriateness of linear regression model was instead rejected.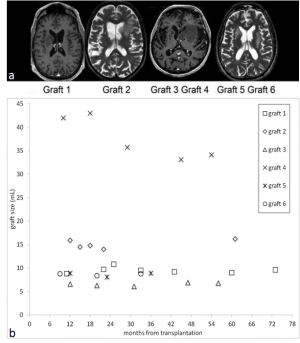
Discussion
The risk of uncontrolled proliferation is intrinsic to cell-based therapy.9 However, to the best of our knowledge, death attributed to fetal graft overgrowth occurred only in two patients with Parkinson’s disease.10,11 In one patient the striatal and intraventricular grafts were contaminated with tissue from multiple germ layers and the solid intraventricular tissue led to ventricular obstruction.10 In the other patient the midbrain graft was contaminated with choroid plexuses and death was due to mass effect created by a cystic lesion.11 In one patient with Huntington’s disease, who received bilateral intrastriatal transplants of lateral ganglionic eminence from several fetuses, multiple solid and one ependymal cystic mass lesions were discovered five years after transplantation, but both remained stable for four years until death which followed complications of disease progression.12
In the four patients reported here, a less pronounced motor and cognitive decline without any symptom or sign of mass effect was demonstrated.4 The results of the present investigation indicate that the large size attained by grafts in these patients is not followed by and does not imply progressive uncontrolled growth. This is in line with [18F]fluorodeoxyglucose positron emission tomography data in the same patients, showing striatal and cerebral cortical metabolic increase compared to the pre-surgical evaluation two years after transplantation.4,7 The slight relative metabolic decrease observed at later follow-up4 would be inconsistent with a neoplastic drift of the grafts. The biological mechanisms regulating the development of fetal striatal tissue into a diseased brain deserve as much understanding as the clinical impact of this growth on the transplanted patients. Sarchielli et al.13 extensively characterized primary cell cultures from human fetal striatal primordium. These cells express neurotrophins able of maintaining cell plasticity, as well as promoting neurogenesis, migration and survival.13 Moreover, Gallina et al.14 recently showed that also in a graft of gestational age comparable with those of fetuses used as source in the present cases, cells exhibit a surface biomarker code, namely CD15, CD24, and CD29, which regulates neural cell proliferation and differentiation.15 Overall, available evidence is consistent with the view that the large size attained by some grafts in Huntington’s disease transplantation is the result of a time-scheduled and self-limiting development of the striatal primordium.14 We are conscious that present knowledge is not sufficient to ensure that these grafts will maintain their quiescent status and, therefore, further surveillance is warranted.
Competing Interests
The authors have declared that no competing interests exist.
Acknowledgements
We thank Dr. Stefano Chiti for his skilful technical assistance. Authors’ contribution was as follows: MM, BP, and PG designed the study and wrote the manuscript with support from NDL. AB and AG cross-checked neurological and imaging data. SD and BP performed statistical analysis. MP, LL, and GBV contributed to conception of the study and critically reviewed the manuscript.Appendix 1
References
- Bachoud-Lévi AC, Gaura V, Brugières P, Lefaucheur JP, Boissé MF, Maison P, Baudic S, Ribeiro MJ, Bourdet C, Remy P, Cesaro P, Hantraye P, Peschanski M. Effect of foetal neural transplants in patients with Huntington's disease 6 years after surgery: a long-term follow-up study. Lancet Neurol 2006; 5: 303-309
- Reuter I, Tai YF, Pavese N, Chaudhuri KR, Mason S, Polkey CE, Clough C, Brooks DJ, Barker RA, Piccini P. Long-term clinical and positron emission tomography outcome of fetal striatal transplantation in Huntington’s disease. J Neurol Neurosurg Psychiatry 2008; 79: 948–951
- Barker RA, Mason SL, Harrower TB, Swain RA, Ho AK, Sahakian BJ, Mathur R, Elneil S, Thornton S, Hurrelbrink C, Armstrong RJ, Tyers P, Smith E, Carpenter A, Piccini P, Tai YF, Brooks DJ, Pavese N, Watts C, Pickard JD, Rosser AE, Dunnett SB; NEST-UK collaboration. The long-term safety and efficacy of bilateral transplantation of human fetal striatal tissue in patients with mild to moderate Huntington’s disease. J Neurol Neurosurg Psychiatry 2013; 84: 657-665
- Paganini M, Biggeri A, Romoli AM, Mechi C, Ghelli E, Berti V, Pradella S, Bucciantini S, Catelan D, Saccardi R, Lombardini L, Mascalchi M, Massacesi L, Porfirio B, Di Lorenzo N, Vannelli GB, Gallina P. Foetal striatal grafting slows motor and cognitive decline in Huntington disease. J Neurol Neurosurg Psychiatry 2013 Dec 17. doi: 10.1136/jnnp-2013-306533. [Epub ahead of print]
- Baizabal-Carvallo JF. Fetal grafting for Huntington’s disease. Is there a hope? J Neurol Neurosurg Psychiatry 2014 Jan 8. doi: 10.1136/jnnp-2013-307252 [Epub ahead of print]
- Cisbani G, Cicchetti F. The fate of cell grafts for the treatment of Huntington’s disease: the post-mortem evidence. Neuropathol. Appl. Neurobiol. 2014; 40: 71-90
- Gallina P, Paganini M, Lombardini L, Mascalchi M, Porfirio B, Gadda D, Marini M, Pinzani P, Salvianti F, Crescioli C, Bucciantini S, Mechi C, Sarchielli E, Romoli AM, Bertini E, Urbani S, Bartolozzi B, De Cristofaro MT, Piacentini S, Saccardi R, Pupi A, Vannelli GB, Di Lorenzo N. Human striatal neuroblasts develop and build a striatal-like structure into the brain of Huntington’s disease patients after transplantation. Exp Neurol 2010; 222: 30-41
- Freeman TB, Cicchetti F, Bachoud-Levi AC, Dunnett SB. Technical factors that influence neural transplant safety in Huntington’s disease. Exp Neurol 2011; 227: 1-9
- Lepore AC, Neuhuber B, Connors TM, Han SS, Liu Y, Daniels MP, Rao MS, Fischer I. Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience 2006; 142: 287-304
- Folkerth RD, Durso R. Survival and proliferation of non-neural tissues, with obstruction of cerebral ventricles, in a parkinsonian patient treated with fetal allografts. Neurology 1996; 46: 1219-1225
- Mamelak AN, Eggerdin FA, Oh DS, Wilson E, Davis RL, Spitzer R, Hay JA, Caton WL 3rd. Fatal cyst formation after fetal mesencephalic allograft transplant for Parkinson’s disease. J Neurosurg 1998; 89: 592-598
- Keene CD, Chang RC, Leverenz JB, Kopyov O, Perlman S, Hevner RF, Born DE, Bird TD, Montine TJ. A patient with Huntington’s disease and long-surviving fetal neural transplants that developed mass lesions. Acta Neuropathol 2009; 117: 329-338
- Sarchielli E, Marini M, Ambrosini S, Peri A, Mazzanti B, Pinzani P, Barletta E, Ballerini L, Paternostro F, Paganini M, Porfirio B, Morelli A, Gallina P, Vannelli GB. Multifaceted roles of BDNF and FGF2 in human striatal primordium development. An in vitro study. Exp Neurol 2014; 257: 130-147
- Gallina P, Paganini M, Biggeri A, Marini M. Romoli AM, Sarchielli E, Berti V, Ghelli E, Guido C, Lombardini L, Mazzanti B, Simonelli P, Peri A, Maggi M, Porfirio B, Di Lorenzo N, Vannelli GB. Human striatum remodeling after neurotransplantation in Huntington’s disease. Stereotact Funct Neurosurgery 2014; 92: 211-217
- Pruszak J, Ludwig W, Blak A, Alavia K, Isacson O. CD15, CD24, and CD29 define a surface biomarker code for neural lineage differentiation of stem cells. Stem Cells 2009; 27: 2928-2940

Leave a Comment
You must be logged in to post a comment.