Abstract
Background: As Pandemic (H1N1) 2009 influenza spreads around the globe, it strikes school-age children more often than adults. Although there is some evidence of pre-existing immunity among older adults, this alone may not explain the significant gap in age-specific infection rates.
Methods & Findings: Based on a retrospective analysis of pandemic strains of influenza from the last century, we show that school-age children typically experience the highest attack rates in primarily naive populations, with the burden shifting to adults during the subsequent season. Using a parsimonious network-based mathematical model which incorporates the changing distribution of contacts in the susceptible population, we demonstrate that new pandemic strains of influenza are expected to shift the epidemiological landscape in exactly this way.
Conclusions: Our results provide a simple demographic explanation for the age bias observed for H1N1/09 attack rates, and a prediction that this bias will shift in coming months. These results also have significant implications for the allocation of public health resources including vaccine distribution policies.
Article Updated
This article has been updated: “The Shifting Demographic Landscape of Pandemic Influenza” in PLOS ONE, doi: 10.1371/journal.pone.0009360. This update is in line with PLOS policy at the time of publication, see here for further details.
Introduction
In March 2009, a new A/H1N1 influenza strain (Pandemic (H1N1) 2009 influenza or H1N1/09) emerged in humans in Mexico and by early June 2009, the World Health Organization (WHO) had raised the worldwide pandemic alert level to signal a global pandemic of a novel influenza virus. Since the WHO declaration of a pandemic, the new H1N1/09 virus has continued spread across the globe, causing growing outbreaks in most countries [1] . Public health officials anticipate that there will be more H1N1/09 cases, hospitalizations and deaths throughout the fall and winter in the northern hemisphere [2] . Early epidemiological data suggest that the 2008-2009 seasonal influenza vaccines are not effective against the new strain but that older individuals who were previously infected by another H1N1 strain may be less likely to develop clinical infection [3] . Multiple manufacturers are now developing vaccines for H1N1/09, and as a best case scenario, the distribution may begin as early as October 2009, until which time the virus will continue to spread.
Influenza is a complex and continually changing disease that infects individuals of all ages. In contrast to diseases like measles and rubella, the dynamics of influenza are strongly influenced by the evolution of immunological properties of the pathogen [4] . The epidemiological landscape of flu is dynamically shaped by cycles of naturally-acquired immunity (through infection) and immune escape (through viral evolution). Based on data from prior influenza pandemics and a simple network-based mathematical model, we argue that, for a newly introduced strain of influenza, this process will cause a shift in the demographic burden of influenza from children to adults. Our analysis also has implications for optimal vaccine allocation. We echo our previous conclusions that high-risk groups should receive highest priority and direct protection when vaccine supplies are limited [5] . However, secondary efforts should focus on indirect protection of groups with greatest potential for infection, whose identities may change over time.
Methods
Population Model
Influenza spreads during close contacts between susceptible and infected individuals. The likelihood of a person becoming exposed to disease will strongly depend on the number and intensity of his or her interactions [6][7][8][9][10] . To study how the interplay of heterogeneities in interaction patterns and infection-induced immunity drive the demographic progression of pandemic influenza, we use network models in which individuals are represented as nodes , and edges connecting nodes represent disease-causing interactions, or contacts , which may occur between individuals during an infectious period. The number of edges for a given node is known as the node’s degree, and the probability distribution of degrees over all nodes is referred to as the degree distribution.
Our network model represents an urban area population and is based on data for the city of Vancouver, British Columbia [7] . We model the interaction patterns relevant for the spread of influenza in the population via a data-driven, activity-based contact network model. Each person is assigned an age based on census data from the city of Vancouver, British Columbia, and age-appropriate activities (e.g. school, work, nursing home, etc.) Contacts among individuals reflect household size, employment, school and hospital data also from Vancouver. (More details can be found in [5][7]). The emergent age-specific contact patterns of our model closely resemble other empirical estimates (Figure 1), and parsimoniously incorporate individual-level heterogeneity.
Modeling Immunity and Second Season Dynamics
We assume that an infected node will infect a susceptible contact with a given probability (known as transmissibility) that depends on both the infectiousness and susceptibility of the nodes. Once infected, a node cannot be reinfected during the same outbreak and will have resistance to infection during the subsequent season (Figure 2). The cross-immunity in the second season is assumed to be partial and we use to represent the loss of immunity from one season to the next (
is full immunity against future infection,
is complete loss of immunity, and intermediate values correspond to partial immunity). For influenza A, natural immunity acquired in one epidemic tends to be heterotypic for the second epidemic appearance of the virus, though several studies have shown that individuals infected by influenza A can be reinfected by antigenically similar strains during the following seasons. An estimated 8% of individuals who were infected in the 1918-1919 Spanish pandemic were reinfected in January-February 1920 [11]; and the relative risk for clinical illness during the second wave of the 1918 pandemic after infection in the first wave was estimated to be as low as 6% in U.S. Army personnel camps (but was found to be as high as 51% in one of the camps)[12]. Similar rates of reinfection have been estimated for the 1968 Hong Kong influenza pandemic [13][14]. Generally, immunity loss for influenza A has been estimated at 5% per year [15], and an estimated 7.4% of previously infected individuals become fully susceptible within one year [16]. Should H1N1/09 produce a second season of transmission, these studies of prior pandemic strains suggest that . will likely lie somewhere between 0.05 and 0.10. Following infection, antibodies that recognize influenza surface antigens, hemagglutinin and neuraminidase, persist and are associated with resistance to reinfection [17]. There is evidence that anti-hemagglutinin (HA) antibodies limit reinfection by antigenically similar strains of influenza [18][19] and anti-neuraminidase (NA) antibodies significantly reduce virus replication and release if reinfected [20][21]. Thus, our model assumes that cross-immunity reduces both susceptibility and infectiousness by a factor
.
Most empirical studies measuring immunity to influenza measure a reduction in infection rate at the population scale. Thus, we model the spread of influenza in a partially immune population assuming perfect partial immunity, using empirical data for infection-acquired immunity to influenza described above. Perfect partial immunity implies that for a level of loss of partial immunity , a proportion
of the previously infected population is completely protected, while the remaining
are fully susceptible. (If empirical data on leaky partial immunity for influenza is available, the network-based leaky immunity model described in [22] can be used instead.)
To consider disease dynamics beyond the initial pandemic period, we have developed a mathematical approach based on percolation methods. The standard bond percolation model [23] assumes no pre-existing immunity, thus is an appropriate model for the spread of a novel influenza strain. Using this method to model the spread of influenza in a naive population, we can define a residual network, which is the relevant contact network for a subsequent outbreak caused by the same pathogen in the same population. The residual network is made up of individuals who were not infected in the initial epidemic, a proportion . of who were infected but have lost immunity since infection and the edges joining them. We describe the residual network via its degree distribution, , or the probability that a (susceptible) individual in the residual network
contacts with other (susceptible) individuals in the residual network (which we derive in Supplementary Information) [22]. Given the transmissibility of the second season pathogen,
, (which may or may not be different than the transmissibility of the .first season pathogen),), and use bond percolation techniques to predict the consequences of a second season spread of infection. The relationship between transmissibility
and the reproductive number in a naive population
is described by:
where is the degree distribution in the naive population. Similarly, the effective reproductive number in the partially immune population
is described by
An individual will be susceptible to infection in the second season if they were not infected in the first season or if they were infected and have lost immunity (with probability ). Thus, the probability that a node of degree
is susceptible to infection in the second season is equal to:
where, is the transmissibility of the first season influenza strain and
is a quantity that can be calculated from bond percolation techniques and depends on both the population’s contact structure as well as the transmissibility of the pathogen [23]. Also, the probability of infection in the second season to an individual of residual degree
(number of edges in the residual network) can be computed as
, where
is the transmissibility of the second season influenza strain, and
is a quantity that can be calculated from bond percolation techniques. We can combine these two quantities to find the risk of infection to a node of (original) degree k in the second season:
where, is the probability that a node will have residual degree
, given that it has a degree of
before the first season (and is derived in Supplementary Information) [22]. The values for risk of infection for the second season shown in Figure 3 were calculated using the above formulation, and verified by comparison to stochastic simulations (not shown). Stochastic simulations for this verification and Figure 3(A) assumed a simple percolation process with
and
as described.
Vaccination Priorities
We modeled vaccination priorities by randomly selecting individuals within the priority group (e.g. school-age children). We modeled cross-immunity from exposure to pre-1957 H1N1 strains of influenza by immunizing a randomly chosen subset of adults (9%) and elderly (33%).
Results
Evidence for a Fluctuating Landscape
Pandemic influenza is feared for its severe excess mortality [24] , rates for which can vary greatly. For most seasonal and pandemic flu, the elderly and very young are at highest risk for severe disease; however, the 1918 Spanish flu pandemic is believed to have been deadliest for 20-40 year olds [25] . influenza morbidity and attack rates also vary among demographic groups. Epidemiological studies and conventional wisdom suggest that school children have the highest attack rates and ultimately fuel transmission throughout the community [26][27][28][29][30] . Data from the three known influenza emergence events in the twentieth century initially show this bias towards school-aged children (Figure 3). When we look beyond the initial pandemic period, however, the age-specific attack rates reverse, with the probability of infection in adults exceeding that of children.
Data from H1N1/09 outbreaks thus far reveals a similar initial discrepancy in attack rates (Figure 3, [31][32] ). There is mounting evidence that cross-immunity from exposure to prior strains may be protecting older adults [3] , as has been suggested for infection with the 1918 influenza strain [33] . However, there is a simple and complementary explanation for the differences in attack rates and subsequent age shifts that is based on the heterogeneous contact patterns underlying the spread of influenza.
Several diverse studies have estimated the distribution of contact patterns among age groups, primarily in urban populations [34][35][36][37][38] . Although the studies use different definitions of contact and contact rate, all but one suggest that children have the highest numbers of contacts followed by adults (Figure 1). Basic epidemiological theory suggests that, in the absence of intervention and cross-immunity, children should therefore have the highest attack rates [7][39][40] . As infection-induced immunity accumulates among the highly connected individuals, however, the infection cascades into other parts of the population [22][41][42] . In fact, stochastic simulations of disease transmission illustrate that even within a single influenza outbreak, the burden of disease shifts from children to adults as disease progresses from the most connected to more moderately connected portions of the population (Figure 4(A)).
Using our mathematical model, we calculate the expected age-specific attack rates in the .first and second seasons given the contact structure of the network (its degree distribution), the infectiousness of the strain, and the level of partial immunity from one season to the next. We .find that the attack rate shifts shown in Figure 3 are a natural outcome of the contact patterns described in Figure 1. Intuitively, the likelihood of becoming infected during the initial phase of the pandemic increases with number of contacts. However, if the strain makes a second appearance, then the relationship between contact patterns and epidemiological risk is altered by immunity acquired during the initial outbreak (Figure 4(B)). When the population is fully susceptible, the highest-degree nodes are most at risk for infection; and thus are likely to be protected against reinfection. In a partially immune population, while individuals with very few contacts maintain low levels of risk, moderately connected individuals become the most vulnerable subset of the population. This transition is expected to be more pronounced if high levels of immunity are maintained by individuals infected during the initial outbreak (Figure 4(B)).
If the reproductive number in the .first season is (as has been estimated for H1N1/09 [31][43]), our model suggests that the returning strain will only invade if it is more transmissible (higher probability of transmission per contact) than the original strain; however, because of a reduced number of contacts among susceptible individuals, its effective reproductive number may be considerably lower than the original strain (Supplementary Information). Higher transmissibility can occur if the pathogen evolves to be more infectious or virulent [44][45] or if the social structure changes to enhance transmission, for example, with the commencement of school or relaxation of social distancing measures. Children tend to have higher numbers of contacts than adults (Figure 4(C)) (who combined make up more than 80% of the population in most developed nations). Thus Figure 4 suggests that the burden of disease is expected to shift from school-age children to adults both during the initial pandemic and between the initial pandemic and the subsequent season, which is consistent with the patterns observed during the three influenza pandemics of the twentieth century (Figure 3).
Implications for Vaccination
The vaccination of school-age children has been suggested as an effective influenza control strategy [28][46][47][48] ; and school-age children are among the U.S. CDC’s H1N1/09 vaccination priority groups [49] . Since school children are thought to be critical transmitters of flu, immunizing them can break potential chains of transmission before they reach the greater community. This strategy, however, hinges on the primacy of school-age children in influenza transmission and the general idea that the likelihood of catching and spreading flu is proportional to one’s number of contacts [5][50][51][52] . However, our study illustrates that naturally-acquired immunity may restructure the population so that the most highly connected individuals are no longer the most vulnerable nor the most likely to transmit infection. Thus the optimal vaccination strategy may depend on the recent epidemiological history of the population. For example, we consider a scenario in which there is a limited supply of vaccine doses (15% coverage) and consider two strategies: (i) vaccinate a random subset of children or (ii) vaccinate a random subset of adults (Figure 5). In other words, vaccinating the most highly connected age group or a more moderately connected age group. This analysis is meant to simply explore whether vaccination strategies to minimize transmission should shift as the disease alters the immunological structure the host population; and we do not consider other outcome measures such as mortality, years of life lost or economic costs [5][53][54] .
Vaccination reduces the size of the epidemic through both direct protection of 15% of the population and indirect protection of others through partial herd immunity. Figure 5(A) shows that during the initial phase of pandemic spread, when the population is fully susceptible, it is more effective to vaccinate children than adults. This prediction reverses for a second season, with adult vaccination more effectively reducing total cases than school-age vaccination. We further consider the impact of resistance from exposure to prior strains of the same subtype among older adults (Figure 5(B)). In particular, there are estimates that 9% of adults and 33% of elderly are resistant to H1N1/09 from H1N1 infections prior to 1957 [3] . Even with historical cross-immunity, adult vaccination is expected to be more effective than school-aged vaccination in the second season.
Discussion
Influenza transmission is constrained by contact patterns, which are influenced by individual behavior and sociological events. For example, the early transmission of H1N1/09 in Mexico City was likely hampered by the closing of schools for the two-week Holy Week period and the subsequent implementation of social distancing interventions including school closures [43] . These events prevented contacts that typically take place within schools that are thought to be pivotal to spread of flu through communities [47][48] . The reverse is also true: the dynamics of infectious diseases can dramatically alter the structure of a host population. Outbreaks of fully immunizing diseases like measles permanently remove cases from the susceptible fraction of the population. influenza, along with many other partially immunizing diseases such as RSV, pertussis and rotavirus, provides temporary incomplete immunity. Individuals fade in and out of the epidemiological active portion of the population as they become infected and slowly regain susceptibility to future infection. When a novel influenza strain emerges into a pandemic, it works its way through the population, preferentially infecting and thus immunizing individuals with high numbers of contacts. It essentially prunes the underlying contact network by removing highly connected individuals and all of their connections. If the strain reemerges in the following season, it faces much sparser chains of susceptible individuals, in which spread is more limited and new groups are expected to bear the brunt of the epidemic. Our simple network-based mathematical model elucidates this phenomenon by both incorporating the heterogeneous distribution of contacts among age groups and tracking the changing immunological structure of the population from one season to the next [10][22] .
This model does not consider demographic processes such as births, deaths, and aging. We have found, however, that population aging has minimal impact on network structure or disease dynamics across levels of immunity (Supplementary Information). We also have not addressed the dynamics beyond two seasons and believe that, while the relative risks will continue to change, we cannot simply extrapolate our results to future seasons. When schools are in session, school children tend to have the highest numbers of contacts among all age groups [34][55] . Consequently, they often form the leading edge of a pandemic [31][56][57] . Adults tend to have lower numbers of contacts and thus lower risk of infection, although they play an important role in spatially dispersing infection [58] . Based on estimated contact patterns, our network model suggests that attack rates for a novel strain of influenza should initially be biased towards children and then shift towards adults. This is consistent with estimated attack rates for the three major pandemics of the 20th century and early H1N1/09 epidemiological data.
This analysis suggests that we might experience a shift in H1N1/09 age-specific infection risks (and thus potential for infecting others) over the next 12 to 24 months, and that the optimal distribution of vaccines and other public health resources may change throughout this period. While many developed nations are aiming for universal vaccination, estimates for the delivery of vaccines range from late September through January 2010 [59][60][61] . As H1N1/09 continues to spread, it is changing the immunological landscape of the population. The most vulnerable groups in September 2009 may look very different than the those in November 2009. For example, if mass school-based transmission of H1N1/09 has occurred prior to the availability of vaccines, then perhaps school-aged children should receive lower priority than other groups and school closures should be de-emphasized as a disease control measure.
Although our study does not explicitly consider the important option of prioritizing groups at high risk for mortality, we echo our previous claim ( [5] ) that high-risk groups and critical personnel should receive highest priority when vaccine supplies are limited. Secondary efforts should focus on groups with greatest potential for becoming infected and infecting others in order to maximize indirect protection. This study suggests that the identities of these high transmission groups depend on the epidemiological history of the population. For seasonal influenza, the high risk age groups (elderly and infants) are distinct from the high transmission age group (school-age children). However, during the second season following a pandemic these priorities may align. In prior pandemics, the highest-risk age groups were young healthy adults (1918) or elderly and infants (1957 and 1968) [62] . Thus, in a partially immune population, prioritizing adults, elderly and infants may not only provide indirect protection by achieving the greatest herd Immunity but also directly protect those at risk of complications or death.
Figures
We compare six estimates for the mean degree by age of individuals (left panel) and the mean degree across the population (right panel). Meyers et al. [7] and Eubank et al. [38] are model-based estimates in which survey, census and other data were used to construct detailed computer simulations of contact patterns in Vancouver, BC and Portland, OR, respectively. The remaining four sets of estimates are inferred from responses to survey questions about the frequencies of (a) two-way conversations lasting three or more words in the physical presence of another individual, and (b) a physical contacts which involve skin-to-skin contact. The Wallinga study [37] includes only conversational contacts, while the Mossong, Read and Beutels studies [34][35][36] include both contact types. The Read and Beutels studies only include adults. Our model (based on [7]) measures contacts during an average infectious period, while the remaining studies measure daily contacts.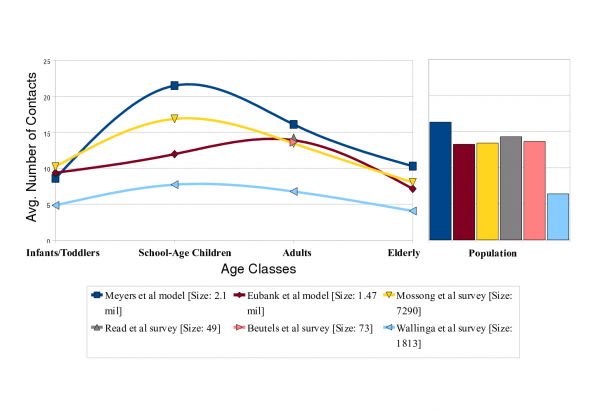
Fig. 1: Estimated age-specific contact rates in an urban population
Lines in these network diagrams indicate contacts through which influenza can spread. Prior to the introduction of a novel pandemic strain, most of the population is susceptible. The pandemic initially sweeps through the most connected portions of the populations, including groups of school-age children, leaving a wake of temporarily immunized individuals. The remaining susceptible population will consist of less connected portions of the population.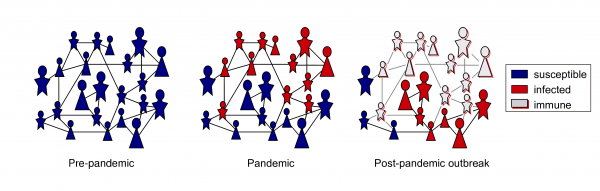
Fig. 2: Changing immunological structure of a population throughout an influenza pandemic
Multiple bars for a single strain represent data from different populations. Data are from a: [57], b: [56], c: [63], d: [64], e: [65], f: [66], g: [67], h:[68], i: [69], j: [70], k: [71], l: [32]. Numbers above bars represent odds ratios. While there are consistent qualitative patterns, the estimates are based on diverse data and methodologies and thus should not be compared quantitatively across studies. The 1968 Hong Kong H3N2 pandemic is the only one of the four strains that does not appear to have an initial bias towards children, which may be influenced by cross immunity from prior H2N2 infections as the two viruses shared nearly identical neuraminidase molecules[72]. Data for H1N1/09 is reported as number of confirmed cases as a proportion of age group size in the respective country.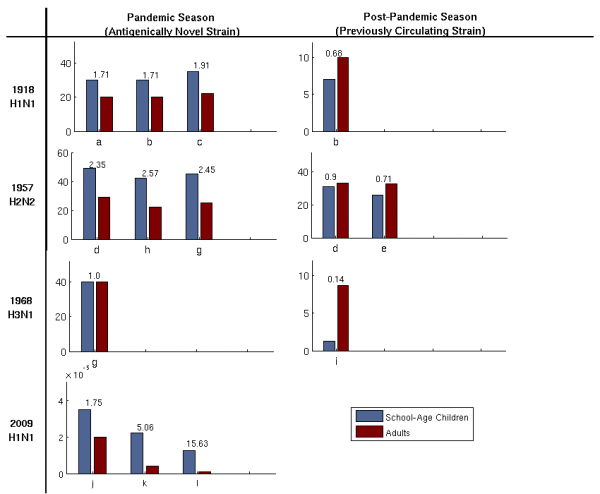
Fig. 3: Attack rates among adults and children during influenza pandemics and subsequent seasons
(A) During the initial pandemic season, we notice a shift in the attack rate (the number of new cases during a week in an age group divided by the size of the age group). The attack rate among children is initially higher than the attack rate among adults, but this reverses after the epidemic peak. (B) During the initial pandemic, all individuals are susceptible, and risk of infection (defined in Methods) increases with number of contacts (dashed brown line, and right y-axis). During a subsequent outbreak the epidemiological risk landscape shifts towards moderately connected individuals, depending on the the level of immunity (green lines, and left y-axis) for T1 = 0:09 (R0 = 1:6) and T2 = 0:15 (Re = 1:05; 1:16). (C) The degree distributions for school-age children (mean degree of 21.5) and adults (mean degree of 16.1) in our urban population network model. The bimodal adult degree distribution reflects heterogeneities in adult employment status.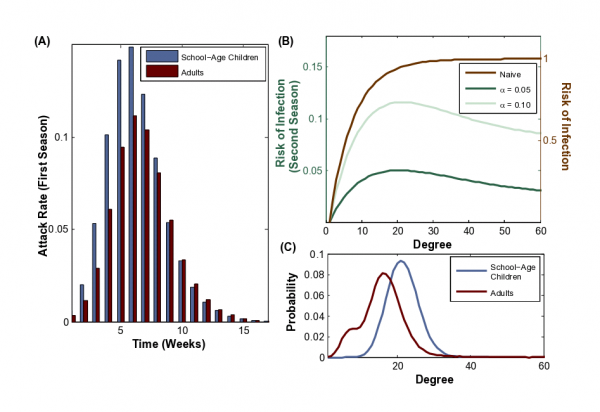
Fig. 4: Individual risk of influenza infection during two sequential outbreaks
(A) The impact of school-aged and adult vaccination priorities at 15% vaccine coverage in a naive (“Season 1”) and partially immune population (“Season 2”) population at alpha = 0.05 (B) The impact of these policies assuming pre-existing resistance among adults (9%) and elderly (33%) acquired through exposure to a strain of the same subtype prior to 1956. The first season pathogen has a reproductive ratio of R_0 = 1.6 and the second season pathogen has an effective reproductive ratio of R_e = 1.005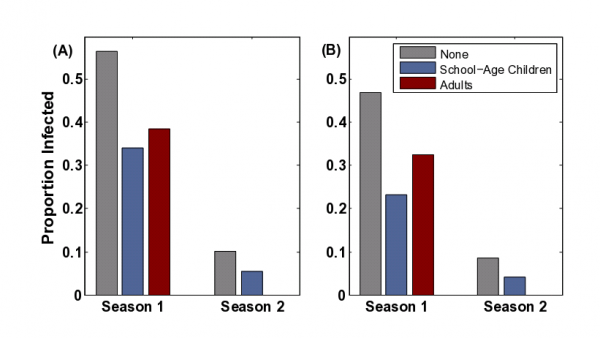
Fig. 5: Comparison of vaccination policies
Acknowledgments
The authors thank the U.S. Centers for Disease Control and Prevention (CDC) Epidemiology/Surveillance Team for sharing aggregate case count data on the H1N1 outbreak in the United States; , and Joel Mossong, Jonathan Read, Phillipe Beutels, and Clement Tuberlin for sharing data; Jessica Metcalf for assistance with data; and Tom Hladish for discussions.
Funding Information
This work was supported by the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health; grants from the James F. McDonnell Foundation, National Science Foundation (DEB-0749097), and NIH Models of Infectious Disease Agent Study (MIDAS) (U01-GM087719-01) to L.A.M; and support from the Canadian Institutes of Health Research (PTL97125 and PAP93425) and the Michael Smith Foundation for Health Research to B.P.
Competing Interests
The authors have declared that no competing resources exist.
References
- Margaret Chan. influenza A(H1N1): lessons learned and preparedness.2009.
- A. Schubert. CDC briefing on investigation of human cases of h1n1 .Press Briefing Transcripts: http://www.cdc.gov/media/transcripts/2009/t090724.htm, pages Accessed Aug 2, 2009, 2009.
- Centers for Disease Control and Prevention. Serum Cross-Reactive Antibody Response to a Novel influenza A (H1N1) Virus After Vaccination with Seasonal influenza Vaccine. Morb. Mortal. Wkly. Rep.,58(19):521-524, May 2009.
- D.J. Smith, A.S. Lapedes, J.C. de Jong, T. M. Bestebroer, G. F. Rimmelzwaan, A. D. M. E. Osterhaus, and R. A. M. Fouchier. Mapping the antigenic and genetic evolution of influenza virus. Science, 305:371-6, 2004.
- S. Bansal, B. Pourbohloul, and L.A. Meyers. Comparative analysis ofinfluenza vaccination programs. PLoS Medicine, 3, 2006.
- L.A. Meyers, M.E.J. Newman, M. Martin, and S. Schrag. Applying network theory to epidemics: control measures for mycoplasma pneumoniae outbreaks. Emerg. Infect. Dis., 9(204), 2003.
- L.A. Meyers, B. Pourbohloul, M.E.J. Newman, D.M. Skowronski, and R.C Brunham. Network theory and sars: predicting outbreak diversity. J. Theo. Biol, 232:7181, 2005. 13
- M.J. Keeling and K.T.D. Eames. Networks and epidemic models. J. R.Soc. Interface, 2:295-307, 2005.
- M.J. Keeling. The effects of local spatial structure on epidemiological invasions. Proc. R. Soc. B, 266:859.867, 1999.
- S. Bansal, B. Grenfell, and L.A. Meyers. When individual behavior matters. J. R. Soc. Interface, 4(16), 2007.
- E.O. Jordan. Epidemic influenza: A survey. American MedicalAssociation, Chicago., 1927.
- JM Barry, C Viboud, and L. Simonsen. Cross-protection between successive waves of the 1918-1919 influenza pandemic: epidemiological evidence from us army camps and from britain. J Infect Dis, 198:1427.34,2008.
- H.M. Foy, M.K. Cooney, and I Allan. Longitudinal studies of type a and b influenza among seattle schoolchildren and familiies. J. Infect. Dis,134:362, 1976.
- G.R. Noble, H. Wu, GE Tegtmeier, PS Clark, TR Bender, and T.D.Y Chin. Hong kong influenza. Am J Epidemiol, 100:517, 1974.
- CM. Pease. An evolutionary epidemiological mechanism, with applications to type a infuenza. Theoretical Population Biology, 31:422-452, 1987.
- BF Finkenstadt, A Morton, and DA Rand. Modelling antigenic drift in weekly incidence. Am Stat, 24:3447-61, 2005.
- RB Couch and JA. Kasel. Immunity to influenza in man. Annu Rev Microbiol, 37:529.549, 1983.
- W.P. Glezen and R Couch. Viral Infections of Humans,Chapter: Influenza Viruses, pages 473.505. Springer, 1997.
- J.L.0 Shulman. Effects of immunity on transmission of influenza:Experimental studies. Progr. Med. Virol., 12:128.16, 1970.
- BR Murphy, JA Kasel, and RM Chanock. Association of serum anti-neuraminidase antibody with resistance to influenza in man. NEJM,286:1329.1332, 1972.
- R. Couch, J.A. Kasel, J.L. Gerin, J.L. Schulman, and E.D. Kilbourne.Induction of partial immunity to influenza by a neuraminidase-speci.c vaccine. Journal of Infectious Diseases, 129:411-20, 1974.
- S. Bansal and L.A Meyers. Modeling the immunological trace of an epidemic on a network. In preparation, 2009.14
- M.E.J. Newman. Spread of epidemic disease on networks. Phys. Rev. E., 66(016128), 2002.
- J. Luk, P. Gross, and W.W. Thompson. Observations on mortality during the 1918 influenza pandemic. Clin Infect Dis, 33::1375-1378, 2001.
- C.C. Dauer and R.E. Ser.ing. Mortality from influenza, 1957-1958 and 1959-1960. Am Rev Respir Dis, 83:15-26, 1961.
- I.M. Longini, J.S. Koopman, A.S. Monto, and J.P. Fox. Estimating household and community transmission parameters of influenza. Am J Epidemiol, 115:736.751, 1982.
- I.M. Longini and M.E. Halloran. Strategy for distribution of influenza vaccine to high-risk groups and children. Am. J. Epidemiol, 161:303-306, 2005.
- L.H. Taber, A. Paredes, W.P. Glezen, and R.B. Couch. Infection with influenza a/victoria virus in houston families. J Hyg Lond, 86:303-313,1982.
- AS Monto and F. Kioumehr. The Tecumseh study of respiratory illness.IX. Occurrence of influenza in the community, 1966-1971. Am J Epidemiol, 102:553-63, 1975.
- W.P. Glezen. Emerging infections: pandemic influenza. Epidemiol Rev, 181:64-76, 1996.
- Christophe Fraser, Christl A. Donnelly, Simon Cauchemez, William P. Hanage, Maria D. Van Kerkhove, T. Deirdre Hollingsworth, Jamie Gri.n, Rebecca F. Baggaley, Helen E. Jenkins, Emily J. Lyons, Thibaut Jombart, Wes R. Hinsley, Nicholas C. Grassly, Francois Balloux, Azra C.Ghani, Neil M. Ferguson, Andrew Rambaut, Oliver G. Pybus, Hugo Lopez-Gatell, Celia M. Alpuche-Aranda, Ietza Bojorquez Chapela, Ethel Palacios Zavala, Dulce Ma. Espejo Guevara, Francesco Checchi,Erika Garcia, Stephane Hugonnet, Cathy Roth, and The WHO Rapid Pandemic Assessment Collaboration. Pandemic potential of a strain of influenza a (h1n1): Early .ndings. Science, 324:1557-1561, 2009.
- H. Nishiura, C. Castillo-Chavez, M. Safan, and G. Chowell. Transmissionpotential of the new influenza a(h1n1) virus and its age-speci.city in japan. Eurosurveillance, 14:22, 2009.
- Yasushi Itoh, Kyoko Shinya, Maki Kiso, Tokiko Watanabe, Yoshihiro Sakoda, Masato Hatta, Yukiko Muramoto, Daisuke Tamura, YukoSakai-Tagawa, Takeshi Noda, Saori Sakabe, Masaki Imai, Yasuko Hatta, Shinji Watanabe, Chengjun Li, and Shinya Yamada. In vitro and in vivocharacterization of new swine-origin H1N1 influenza viruses. Nature, 2009.
- J. Mossong, N Hens, M Jit, P Beutels, and K. et al. Auranen. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Medicine, 5:3, 2008.
- J.M. Read, K.T.D. Eames, and W.J. Edmunds. Dynamic social networks and the implications for the spread of infectious disease. J R Soc Interface, 5(26):1001.1007, 2008.
- P. Beutels, Z. Shkedy, M. Aerts, and P. Van Damme. Social mixing pattern of transmission models of close contact infections. Epidemiol Infect, 134:1158-1166, 2006.
- J. Wallinga, P. Teunis, and M Kretzschmar. Using data on social contacts of estimate age-speci.c transmission parameters for respiratory-spread pathogens. Am J Epidemiol, 164:936.44, 2006.
- S. Eubank. Synthetic data products for societal infrastructures and proto-populations: Data set 2.0. Network Dynamics and Simulation Science Laboratory, Virginia Polytechnic Institute and State University Technical Report, NDSSL-TR-07-003, 2009.
- R.M. Anderson and R.M. May. Infectious diseases of humans. Oxford University Press, London, 1991.
- A. Barbour and D. Mollison. Stochastic Processes in Epidemic Theory, chapter Epidemics and random graphs, pages 86-89. Springer, 1990.
- M. Barthelemy, A. Barrat, R. Pastor-Satorras, and A. Vespignani. Velocity and hierarchical spread of epidemic outbreaks in scale-free networks. Phys. Rev. Lett, 92(178701), 2004.
- M. Ferrari, S. Bansal, L.A. Meyers, and O. Bjornstad. Network frailty and the geometry of herd immunity. Proc. of R. Soc, 273:2743-2748, 2006.
- B. Pourbohloul, A. Ahued, B. Davoudi, R. Meza, L.A. Meyers, D.M. Skowronski, I. Villasenor, P. Kuri, F. Galvan, P. Cravioto, J. Trujillo, D.J. Earn, J. Dusho., D. Fisman, J. Edmunds, N. Hupert, S.V. Scarpino, D.M. Patrick, and R.C. Brunham. Initial human transmission dynamicsof the pandemic (H1N1) 2009 virus in North America. influenza and Other Respiratory Viruses, 3 (5):215-22, 2009.
- J.K. Taubenberger and D.M. Morens. 1918 influenza: the mother of all pandemics. Emerg Infect Dis, 12(1):15-22, 1996.
- J. Nguyen-Van-Tam and A.W. Hampson. The epidemiology and clinical impact of pandemic influenza. Vaccine, 21:1762-1768, 2003.
- I.M. Jr. Longini, M.E. Halloran, A. Nizam, and Y. Yang. Containing pandemic influenza with antiviral agents. Am J Epidemiol, 159:623-633, 2004.
- T.A. Reichart, N. Sugaya, D.S. Fedson, W.P. Glezen, and L. Simonsen. The japanese experience with vaccinating school-children against influenza. N Engl J Med, 344:889-896, 2001.
- A.S. Monto, J.S. Koopman, and I.M. Jr. Longini. The Tecumseh study of illness. XIII. influenza infection and disease. Am J Epidemiol, 121:811-822, 1985.
- Advisory Committee on Immunization Practices, CDC. Novel H1N1 Vaccination Recommendations. http://www.cdc.gov/h1n1.u/vaccination/acip.htm, page Accessed August 5, 2009.
- R. Pastor-Satorras and A. Vespignani. Immunization of complex networks. Phys Rev E, 65:036104, 2001.
- Z. Dezso and A.-L. Barabasi. Halting viruses in scale-free networks. Phys Rev E, 65:055103, 2001.
- M.E. Woolhouse, C. Dye, J.F. Etard, T. Smith, and J.D. Charlwood. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. PNAS, 94:338-342, 1997.
- J. Medlock and A. Galvani. Optimizing influenza vaccine distribution.Science, page 1175570, 2009.
- M. Meltzer, N. Cox, and K. Fukuda. The economic impact of pandemic influenza in the united states: Priorities for intervention. Emerg Infect Dis, 5:659-71, 1999.
- J. Wallinga, W.J. Edmunds, and M. Kretzschmar. Perspective: human contact patterns and the spread of airborne infectious diseases. Trends Microbiol, 7:372-377, 1999.
- W.H. Frost. Statistics of influenza morbidity: with special reference to certain factors in case incidence. Public Health Reports, 36:584-597, 1920.
- S.D. Collins. influenza in the united states, 1887-1956. Public Health Monograph, 48, 1957.
- C. Viboud, O.N. Bjornstad, D. Smith, L. Simonsen, M.A. Miller, and B.T. Grenfell. Synchrony, waves, and spatial hierarchies in the spread of influenza. Science, 312:447-51, 2006.
- World Health Organization. Update on A(H1N1) pandemic and seasonal vaccine availability. July 7, 2009 (Accessed August 5, 2009).
- Centers for Disease Control and Prevention. Preparing for Vaccination with Novel H1N1 Vaccine. http://www.cdc.gov/h1n1.u/vaccination/provider/preparing.htm, July 31, 2009 (Accessed Aug 5, 2009).
- T. Randall. Pandemic shot may be available as early as september. Bloomberg News, July 29,2009 (Accessed Aug 5, 2009).
- L. Simonsen, MJ. Clarke, LB Schonberger, NH Adren, NJ Cox, and K. Fukuda. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis, 178:53-60, 1998.
- S.D. Collins. Age and sex incidence of influenza and pneumoniae morbidity and mortality in the epidemic of 19289-1929 with comparative data for teh epidemic of 1918-1919. Public Health Reports, 46:1909-36, 1931.
- V Gilbert, C. Caraway, J. Bruce, and W. Mogabgab. Epidemic recurrenceof asian influenza in lousiana, 1959-1960. Am J Public Health, 52:1432-43,1962.
- G. Denniston. "influenza surveillance". Technical Report 70, Communicable Disease Center, 1962.
- GS Perrott and FE. Linder. Data on acute upper respiratory diseases.Public Health Rep., 73(2):121-8, 1958.
- L. Davis, G. Caldwell, R. Lynch, R.E. Bailey, and T. Chin. Hong kong influenza: the epidemiologic features of a high school family study analyzed and compared with a similar study during the 1957 asian influenza epidemic. Am J Epidemiol, 92:240-7, 1970.
- R. Gani, H. Hughes, D. Fleming, T. Gri.n, J. Medlock, and S. Leach. Potential impact of antiviral drug use during influenza pandemic. Emerg Infect Dis, 11 (9), 2005.
- M.P Taylor. influenza 1969-1970. J. R Coll. Gen. Practit., 21 (17), 1971.
- Secretaria de Salud. Estadisticas influenza. Accessed June 15, 2009.
- U.S. Centers for Diease Control and Prevention (CDC), Epidemiology/Surveillance Team. S-oiv case count by age strata.Complete Line List, May 19, 2009.
- J.L. Schulman and E.D. Kilbourne. Independent variation in nature of hemagglutinin and neuraminidase antigens of influenza virus. PNAS,63:326-33, 1969.
Leave a Comment
You must be logged in to post a comment.