Abstract
The role of badgers in the transmission and maintenance of bovine tuberculosis (TB) in British cattle is widely debated as part of the wider discussions on whether badger culling and/or badger vaccination should play a role in the government’s strategy to eradicate cattle TB. The key source of information on the contribution from badgers within high-cattle-TB-incidence areas of England is the Randomised Badger Culling Trial (RBCT), with two analyses providing estimates of the average overall contribution of badgers to confirmed cattle TB in these areas. A dynamical model characterizing the association between the estimated prevalence of Mycobacterium bovis (the causative agent of bovine TB) among badgers culled in the initial RBCT proactive culls and the incidence among sympatric cattle herds prior to culling is used to estimate the average overall contribution of badgers to confirmed TB herd breakdowns among proactively culled areas. The resulting estimate based on all data (52%) has considerable uncertainty (bootstrap 95% confidence interval (CI): 9.1-100%). Separate analyses of experimental data indicated that the largest estimated reduction in confirmed cattle TB achieved inside the proactive culling areas was 54% (overdispersion-adjusted 95% CI: 38-66%), providing a lower bound for the average overall contribution of badgers to confirmed cattle TB. Thus, taking into account both results, the best estimate of the average overall contribution of badgers is roughly half, with 38% being a robustly estimated lower bound. However, the dynamical model also suggested that only 5.7% (bootstrap 95% CI: 0.9-25%) of the transmission to cattle herds is badger-to-cattle with the remainder of the average overall contribution from badgers being in the form of onward cattle-to-cattle transmission. These estimates, confirming that badgers do play a role in bovine TB transmission, inform debate even if they do not point to a single way forward.
Funding Statement
The Randomised Badger Culling Trial (RBCT) in England was designed, overseen and analysed by the Independent Scientific Group on Cattle TB (John Bourne, Christl Donnelly, David Cox, George Gettinby, John McInerney, Ivan Morrison and Rosie Woodroffe). The RBCT was funded by the Department of Environment, Food and Rural Affairs (Defra) with the cooperation of the many farmers and land occupiers in the trial areas who allowed the experimental treatments to operate on their land. CAD acknowledges the MRC for Centre funding support. The research leading to these results received funding from the European Union Seventh Framework Programme [FP7/2007–2013] under Grant Agreement nu278433-PREDEMICS.Introduction
Wildlife reservoirs make the control of infectious diseases particularly challenging. Badgers (Meles meles) are a wildlife host for Mycobacterium bovis, the causative agent of tuberculosis (TB) in cattle1. Various badger-culling strategies have been used in Great Britain in attempts to reduce M. bovis transmission and to stop the spread of disease1. Annually repeated widespread ‘proactive’ culling, using cage-trapping and shooting, has been shown to reduce confirmed TB incidence in cattle herds within culled areas, but it has also been shown to increase risk among cattle herds on land outside, but within 2 km of, the culling area while culling was ongoing2,3,4,5,6. The disease system is complex, with transmission occurring both within and between species. Against this background policymakers and stakeholders have to weigh the potential benefits of particular disease control measures against their economic costs as well as other factors (including, for example, humaneness).

Badger (Meles meles) – Photo credited to Richard Yarnell.
Current approaches to limiting the contribution badgers make to cattle disease in Great Britain vary by region. In England, badger culling has been licensed; with two pilot culls currently underway7. These pilots are the first legal badger culls since 2005 (when the final proactive culls took place in the Randomised Badger Culling Trial, RBCT). In Wales, badgers are vaccinated (using an injectable vaccine) within a so-called Intensive Action Area8. Scotland is Officially TB Free and post-movement testing of cattle has been in place since 2005 to supplement statutory pre-movement TB testing and further reduce risks from the inward movement of cattle infected with M. bovis9. Vaccine development is ongoing, with oral vaccination of badgers and injectable vaccination of cattle both being potential disease control options in the future.
Debate continues over whether or not any form of badger culling should be included in policies to control cattle TB in Great Britain. Although the debate is multi-faceted involving risk assessment, cost effectiveness, animal welfare, health and safety and ethics, much of the discussion has related to the potential level of benefits that might be achieved by a particular badger culling or vaccination policy. The RBCT provided clear evidence that annually repeated, widespread culling (termed ‘proactive’ culling) could significantly reduce confirmed cattle TB incidence within culled areas, while significantly increasing confirmed cattle TB incidence up to 2 km outside the culled areas while culling was ongoing2,3,4,5,6. The RBCT also provided evidence that a strategy of small localized culls (termed ‘reactive’ culling) could significantly increase cattle TB incidence across the 100 km2 areas randomized to this form of culling10,11.
The percentage of transmission to cattle herds, in a particular area, that is badger-to-cattle (whether transmitted directly or via bacterial contamination of the shared environment) determines the potential for badger-focussed control strategies to limit transmission to cattle but in a non-linear manner because substantial reductions in badger-to-cattle transmission over large areas would reduce onward transmission from cattle to other cattle (and thus from cattle back to badgers). Thus, the overall contribution of badgers to cattle TB is greater than the percentage of transmission to cattle herds that is badger-to-cattle.
Donnelly and Hone12 characterized the association between the estimated prevalence of M. bovis among badgers culled in the initial proactive culls in the RBCT and the incidence of confirmed TB among sympatric cattle herds prior to culling. The best-fitting model included frequency-dependent transmission between cattle herds with badger-to-herd transmission proportional to the estimated prevalence of M. bovis infection among the culled badgers. Inherent in this model was an estimate of the herd incidence level in the absence of transmission from badgers: 3.4% of herds (profile-likelihood-based 95% CI: 0 – 6.7%) would be expected to have TB infection newly detected per annum12, suggesting that herd-to-herd transmission was enough to sustain transmission in the absence of transmission from badgers. However, it was not possible to reject the null hypothesis that herd-to-herd transmission alone was insufficient to sustain bovine TB incidence in the cattle population (p=0.18)12.
Calculations subsequently undertaken based on the best-fitting model yielded an estimate (of roughly 50%) for the average overall contribution of badgers to confirmed cattle TB incidence in the RBCT areas (prior to culling)13. This estimate has informed discussions on limiting risks of badger transmission, but it lacked a confidence interval and sensitivity analysis. This paper provides further analysis of this estimate, its precision and its sensitivity.
Furthermore, this paper reviews the relevance of previously estimated reductions in confirmed cattle TB risk associated with proactive culling observed inside the RBCT culling areas and their implications for the average overall contribution of badgers to confirmed cattle TB.
Methods
The RBCT quantified the impact of badger culling on TB incidence in cattle herds in areas of England where bovine TB is endemic. The RBCT compared the incidence of TB in cattle under three randomly allocated strategies – annually repeated widespread (‘proactive’) culling, localized (‘reactive’) culling around farms with confirmed TB breakdowns and no culling (‘survey-only’) – each replicated 10 times in 100 km2 trial areas recruited as matched sets of three areas, known as ‘triplets’3.
Donnelly and Hone model
The badger data analysed by Donnelly and Hone12 related to badgers culled in the initial proactive culls in the RBCT (excluding 19 badgers with incomplete data), as initially published by Woodroffe et al.14 (Table 1). The cattle herd incidence data analysed related to the 12-month periods preceding the initial proactive badger culls, as initially published by Donnelly et al.2 in the form of Supplementary Data based on location data as recorded in the VetNet database and reproduced by Donnelly and Hone12 (Table 1). It was critical that the cattle data related to a period of time before the initial proactive badger culls to avoid any impact of badger culling on the data analysed.
Data on TB incidence among cattle herds in RBCT areas randomized to proactive badger culling (in the 12 months preceding the initial proactive cull) and on badgers culled in the initial proactive badger culls in the RBCT, as originally published by Woodroffe et al.14 and Donnelly et al.2 and analysed by Donnelly and Hone12. Note: a herd was said to experience a new ‘confirmed’ TB herd breakdown if one or more TB test-positive cattle (reactors) were identified while the herd was not under TB restrictions and post-mortem examination led to the detection of typical bovine TB lesions or culture of M. bovis in at least one reactor.
1 Based on the numbers of total herds and confirmed TB breakdown herds in the 12-month periods preceding the initial proactive badger culls, as initially published by Donnelly et al.2 in the form of Supplementary Data based on location data as recorded in the VetNet database.
2 Based on the numbers of badgers culled in initial proactive culls (excluding 19 with incomplete data), as initially published by Woodroffe et al.14
3 Those badgers culled in initial proactive culls which tested positive for M. bovis, as initially published by Woodroffe et al.14
Triplet
Confirmed new herd breakdowns detected in the 12 months preceding the initial proactive cull 1
Total herds 1
Badgers culled 2
Infected badgers culled 3
A
8
71
55
8
B
15
152
238
13
C
8
105
243
4
D
11
97
293
102
E
4
116
602
29
F
4
138
446
13
G
7
245
422
29
H
11
63
161
12
I
15
100
218
82
J
8
114
442
65
The best-fitting model analysed by Donnelly and Hone12 included frequency-dependent between-herd transmission such that:
where cattle herds transition between states U (uninfected), I (infected, and equivalently infectious, but undiagnosed) and M (under movement controls and thus assumed not infectious to other herds). U, I and M are the numbers of herds, rather than individual cattle, in each of these states and N is the total number of herds (N=U+I+M). Between-herd risk per annum is represented by β and the rate of infection from badgers per annum is represented by α IB/NB where IB denotes the number of infected badgers culled in the initial proactive cull in an RBCT area and NB denotes the total number of badgers culled in the initial proactive cull in an RBCT area. The per-annum rate at which infected herds are detected, and thus put under movement controls, is represented by c. The average time on movement control in years is represented by p. This model, like that of Barlow et al.15, assumed that any cattle-to-badger transmission events were negligible. This assumption is discussed in more detail below.
At equilibrium I* = M*/cp where I* and M* are at their equilibrium values. I* was obtained, as a function IB/NB, from the solution of the following quadratic equation (equation 16 of 12):
As before, p was assumed to equal to 0.7 years (255 days) and
where s is the sensitivity of the herd test and b is the interval between routine herd tests (1 year for all herds in RBCT areas)12. The parameter s is assumed to equal 0.9 unless otherwise noted.
As in Donnelly and Hone12, estimates for α and β were obtained by maximum likelihood based on the log likelihood given by
ignoring the additive constant where B is the number of herd breakdowns in a one-year period among N herds.
In this paper the confidence intervals for α and β were obtained by bootstrapping. This is in contrast to the earlier use of profile likelihoods to obtain confidence intervals12. A benefit of bootstrapping is that confidence intervals can be obtained easily for other quantities, including non-linear functions of α and β. This includes the proportion of herds expected to have TB infection newly detected in a year in the absence of transmission from badgers given by:
where π(q) denotes the proportion of herds expected to have TB infection newly detected in a year in areas in which M. bovis prevalence in badgers is q. The average overall contribution of badgers to confirmed cattle TB incidence in the RBCT areas (prior to culling) was thus obtained by averaging the triplet-specific estimates of this proportion:
The dynamical model also provides an expression for the percentage of transmission to cattle herds that is badger-to-cattle:
based on Equation 1 at equilibrium. The average percentage was similarly obtained by averaging the triplet-specific estimates of this percentage.
Mathematically there is no impact on any of the parameter estimates or maximum likelihood fit of incomplete diagnostic sensitivity in badgers (sB) of the standard necropsy undertaken in the RBCT16, except for a reinterpretation that α = α*/sB, assuming that sB was identical for in all triplets analysed.
For each estimate (including for (I*c)/N, the expected value of B given IB/NB), bootstrap 95% confidence intervals were obtained using two alternative methods. Non-parametric bootstrap samples were obtained by the sampling with replacement of the 10 RBCT areas. Parametric bootstrap samples were obtained with binomial resampling of the observed proportions (B/N) and (IB/NB) for each area. In each case 50,000 bootstrap samples were obtained for each assumed value of s (the sensitivity of the herd test). Non-parametric bootstrap confidence intervals, which are generally wider and therefore more conservative, are reported in the text; however, both types of bootstrap confidence intervals are reported in the tables to allow the reader to compare these alternative methods of quantifying parameter uncertainty. In addition, the estimated values associated with individual bootstrap samples can be plotted to show the bivariate distributions of two estimates or the univariate distributions of single estimates.
A potential criticism of the Donnelly and Hone12 model was that it made the simplifying assumption that cattle-to-badger transmission events were negligible within the context of the model. This may have been a particular issue for the analysis of data from the initial proactive culls undertaken in 2002 after the 2001 epidemic of foot-and-mouth disease (triplets D, I and J). It is known that the delayed testing and removal of TB-affected cattle caused by the FMD epidemic was associated with a significant rise in M. bovis prevalence in badgers17 as well as an increase in the incidence of confirmed TB breakdowns per herd. Thus, an alternative set of estimates was obtained excluding data from triplets D, I and J.
Estimation of the impact of proactive culling on confirmed TB incidence in cattle
Jenkins et al.5 used log-linear Poisson regression to compare the numbers of confirmed herd breakdowns recorded inside trial areas subjected to the proactive culling and inside trial areas with no culling (survey-only). The regression models adjusted for triplet, the log of the number of baseline herds at risk, and the log of the number of confirmed breakdowns recorded in a three-year period before RBCT culling. Confidence intervals were conservatively adjusted for extra-Poisson overdispersion by using an adjustment factor (the square root of the model deviance divided by the degrees of freedom) in all cases where its value was greater than 1.
Because proactive culling continued until 2005, the increased M. bovis prevalence among badgers observed in 200217 is unlikely to have directly affected the estimated impact of proactive culling in the post-trial period (which began in 2006). Thus, the estimate presented is based on data from all 10 RBCT triplets.
Results
Donnelly and Hone model
Assuming that diagnostic sensitivity of the herd test (s) was equal to 0.9, the maximum likelihood estimates of α and β were 0.047 (bootstrap 95% CI: 0.009 – 0.175) and 1.98 (bootstrap 95% CI: 1.57 – 2.12), respectively (Figures 1A, 2A, 2C, Table 2). Based on this model, 3.4% (bootstrap 95% CI: 0 – 8.5%) of herds would have been expected to have TB infection newly detected in a year in the absence of transmission from badgers. This corresponds to an average overall contribution of badgers to confirmed cattle TB of 52% (bootstrap 95% CI: 9.1%-100%) in the RBCT areas (prior to culling) (Figures 3A, 4A, 5A, 5C). The estimated average percentage of transmission to cattle herds that was badger-to-cattle was 5.7% (bootstrap 95% CI: 0.9-25%) (Figures 3C, 4C, 5A, 5C). These estimates and their precision varied little as a function of the assumed level of diagnostic sensitivity of the herd test (for s= 0.7, 0.8, 0.9 and 1; see Table 2). Furthermore, the maximized log likelihood varied little as a function of the assumed level of sensitivity.
Estimates of α; β; the percentage of herds expected to have TB infection newly detected in a year in the absence of transmission from badgers (denoted π(0)); the average overall contribution of badgers to confirmed cattle TB incidence in the RBCT areas (prior to culling); and the average percentage of transmission to cattle herds that is badger-to-cattle with non-parametric [NP] and parametric [P] bootstrap 95% confidence intervals.
Dataset
Herd
test
sensitivity (s)α
β
π(0)
Average overall contribution of badgers to confirmed cattle TB incidence
Average percentage of transmission to cattle herds that was badger-to-cattle
Maximized
log
likelihood
All 10 triplets
0.7
0.060
NP (0.012, 0.229)
P (0.013, 0.168)1.32
NP (0.97, 1.43)
P (1.17, 1.39)3.4%
NP (0%, 8.5%)
P (0%, 6.7%)52%
NP (9.2%, 100%)
P (14%, 100%)7.1%
NP (1.2%, 31%)
P (1.9%, 18%)-316.76
All 10 triplets
0.8
0.053
NP (0.010, 0.199)
P (0.011, 0.146)1.62
NP (1.25, 1.75)
P (1.46, 1.70)3.4%
NP (0%, 8.5%)
P (0%, 6.7%)52%
NP (8.9%, 100%)
P (14%, 100%)6.3%
NP (1.0%, 27%)
P (1.6%, 16%)-316.75
All 10 triplets
0.9
0.047
NP (0.009, 0.175)
P (0.010, 0.129)1.98
NP (1.57, 2.12)
P (1.81, 2.07)3.4%
NP (0%, 8.5%)
P (0%, 6.7%)52%
NP (9.1%, 100%)
P (14%, 100%)5.7%
NP (0.9%, 25%)
P (1.5%, 14%)-316.74
All 10 triplets
1
0.043
NP (0.008, 0.155)
P (0.009, 0.116)2.42
NP (1.97, 2.57)
P (2.22, 2.51)3.5%
NP (0%, 8.4%)
P (0%, 6.8%)52%
NP (9.2%, 100%)
P (14%, 100%)5.2%
NP (0.9%, 22%)
P (1.3%, 13%)-316.74
Omitting triplets
D, I and J0.9
0.045
NP (0.000, 1.69)
P (0.000, 1.25)1.99
NP (0.00, 2.07)
P (0.00, 2.09)3.6%
NP (0%, 6.9%)
P (0%, 7.3%)42%
NP (0.0%, 100%)
P (0.0%, 100%)3.7%
NP (0.0%, 100%)
P (0.0%, 100%)-210.67
If data from triplets D, I and J were excluded from the analysis, the maximum likelihood estimates of α and β were 0.045 (bootstrap 95% CI: 0.000 – 1.69) and 1.98 (bootstrap 95% CI: 0.00 – 2.07), respectively (Figure 1B, 2B, 2D, Table 2). Based on this model, 3.6% (bootstrap 95% CI: 0 – 6.9%) of herds would have been expected to have TB infection newly detected in a year in the absence of transmission from badgers. This corresponds to an average overall contribution of badgers to confirmed cattle TB of 42% (bootstrap 95% CI: 0.0%-100%) in the RBCT areas (prior to culling) (Figures 3B, 4B, 5B, 5D). The estimated average percentage of transmission to cattle herds that was badger-to-cattle was 3.7% (bootstrap 95% CI: 0.0-100%) (Figures 3D, 4D, 5B, 5D).
The black dots represent observed data: badger prevalence data are from badgers culled in the initial proactive culls in the RBCT and the cattle herd incidence data analysed relate to the 12-month periods preceding the initial proactive badger culls. The predicted line (I*c/N) was obtained assuming 90% herd test sensitivity for all ten RBCT triplets (panel A) and excluding triplets D, I and J (panel B). Pointwise 95% confidence limits were obtained using non-parametric (NP) and parametric (P) bootstrapping.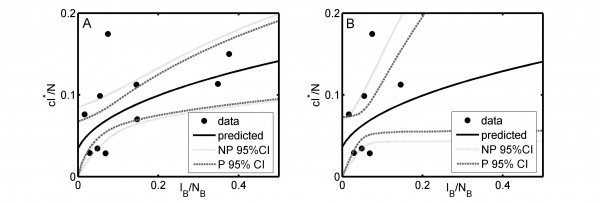
Fig. 1: The proportion of herds in which confirmed cattle TB is newly detected within a year as a function of the observed prevalence of infection in badgers I_B/N_B.
The background depicts the likelihood surface obtained from the original dataset, including all ten RBCT triplets (panels A and C) and excluding triplets D, I and J (panels B and D). The magenta vertical lines represent the critical values β = c. If estimates of β are less than or equal to c, then π(0)=0 and the overall contribution of badgers to confirmed cattle TB is 100%.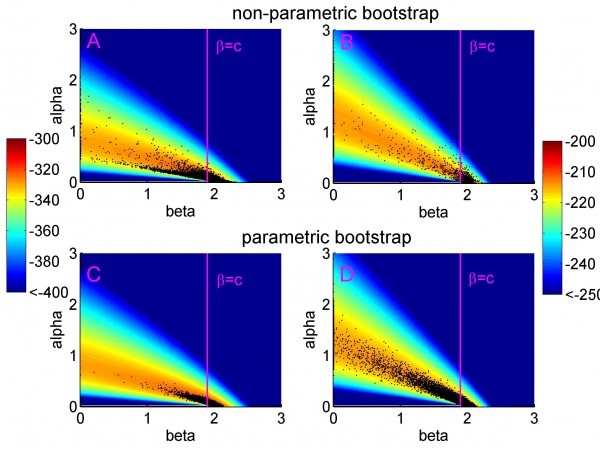
Fig. 2: The bivariate distributions of the estimates of alpha and beta (black dots) obtained from non-parametric (panels A and B) and parametric (panels C and D) bootstrap sampling.
The univariate distributions of bootstrap estimates are based on all ten RBCT triplets (panels A and C) and excluding triplets D, I and J (panels B and D). Note that panels A and B have broken vertical axes.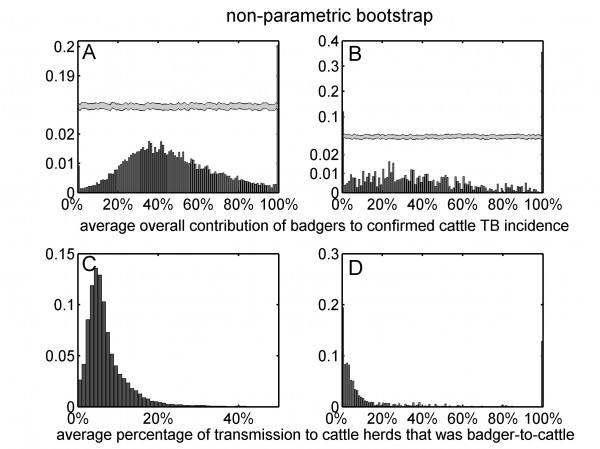
Fig. 3: The univariate distributions of the average overall contribution of badgers to confirmed cattle TB incidence (panels A and B) and the average percentage of transmission to cattle herds that was badger-to-cattle (panels C and D) obtained from non-parametric bootstrap sampling.
The univariate distributions of bootstrap estimates are based on all ten RBCT triplets (panels A and C) and excluding triplets D, I and J (panels B and D). Note that panels A and B have broken vertical axes.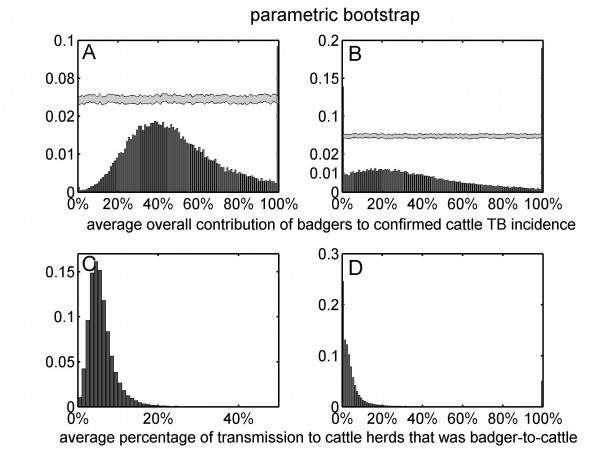
Fig. 4: The univariate distributions of the average overall contribution of badgers to confirmed cattle TB incidence (panels A and B) and the average percentage of transmission to cattle herds that was badger-to-cattle (panels C and D) obtained from parametric bootstrap sampling.
The colour scale corresponds to the proportion of bootstrap estimates that fell within the 5%-by-5% wide bins. The bivariate distributions were obtained from non-parametric (panels A and B) and parametric (panels C and D) bootstrap sampling, including all ten RBCT triplets (panels A and C) and excluding triplets D, I and J (panels B and D).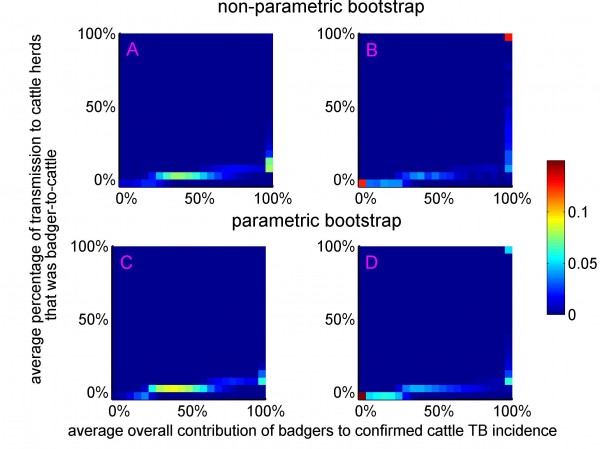
Fig. 5: The bivariate distributions of the average overall contribution of badgers to confirmed cattle TB incidence and the average percentage of transmission to cattle herds that was badger-to-cattle.
Estimation of the impact of proactive culling on confirmed TB incidence in cattle
The largest reduction in confirmed cattle TB incidence achieved over the course of the RBCT was that within RBCT trial areas during the first 18-month time period post-trial (that is between 12 and 30 months after the final annual proactive cull in each of the 10 proactive trial areas). The estimated reduction over this period was 54% with an overdispersion-adjusted 95% confidence interval of 38% to 66%5. Because badgers were never completely eliminated from any of the culling areas and farmers moved cattle in and out of the areas, the lower confidence bound of this estimate can provide a lower bound for the average overall contribution of badgers to confirmed cattle TB in the RBCT areas.
Discussion
The confidence intervals obtained using bootstrap sampling were wider than the profile-likelihood-based intervals previously reported12. However, it was the exclusion of data from triplets D, I and J that considerably widened the confidence intervals obtained. Indeed, the confidence intervals obtained based on the restricted dataset for the average overall contribution of badgers to confirmed cattle TB incidence and the average percentage of transmission to cattle herds that was badger-to-cattle spanned from 0 to 100%.
Taking into account all of the available scientific evidence from all 10 triplets in the RBCT, the best estimate of the average overall contribution of badgers to confirmed cattle TB within high-cattle-TB-incidence areas is roughly half, with 38% being a robustly estimated lower bound and no robustly estimated upper bound.
Although an average overall contribution from badgers of at least 38% indicates their importance in the disease system (at least within the areas analysed), it does not indicate how this contribution might best be limited. Badger vaccination with injectable BCG (in Wales) and badger culling (England) are both currently underway as part of government policies. The former was adopted based on evidence that injectable badger vaccines reduce disease in badgers18, and the latter was adopted based on evidence from the RBCT that annually repeated widespread badger culls over large areas can yield net reductions in cattle herd incidence in the affected area (the culling area and up to 2 km outside of it)2,3,4,5,6. However, it should be noted that the controlled shooting of free-ranging badgers, allowed in the pilot badger culls currently underway in England, was not part of the RBCT (which relied on shorter bouts of cage trapping badgers before they were culled).
The 54% reduction (overdispersion-adjusted 95% confidence interval: 38% to 66%) in confirmed cattle TB incidence was only within RBCT trial areas for an 18-month time period between 12 and 30 months after the final annual proactive cull in each of the 10 proactive trial areas, following an average of 5 years of proactive culling. Long-term estimates (over 9.5 years including 5 years of culling) of the average net impact of proactive culling of circular areas of 150km2were 3–22% (central figure 12%) or 8–24% (central figure 16%), depending on particular assumptions19. At present there is no evidence for the impact of badger vaccination on TB incidence in cattle because no study to measure the impact has been conducted. However, there is evidence from a field trial that badger vaccination significantly reduced the risk of vaccinated badgers testing positive to the available live tests of infection20,21.
According to the analyses presented, although a complete absence of transmission from badgers from the system is predicted to halve the incidence of TB in cattle (with a 38% reduction being a robustly estimated lower bound), only 5.7% (bootstrap 95% confidence interval: 0.9%-25%) of transmissions to herds were estimated to have been badger to cattle. However, the data analysed related to initial proactive culls in the RBCT, which took place between 1998 and 2002. Since this time, statutory pre-movement TB testing of cattle was introduced to reduce risks from the inward movement of cattle infected with M. bovis. By reducing herd-to-herd transmission events, pre-movement testing would likely have increased both the overall contribution of badgers to confirmed cattle TB incidence and the percentage of transmission to cattle herds that was badger-to-cattle.
Clearly derived statistical estimates are very important in a debate where some stakeholders maintain that badgers do not transmit TB to cattle, while others claim that the majority of cattle infections are acquired directly from badgers. Our findings show that badgers play a major role in maintaining M. bovis infection in England’s high incidence areas, but also indicate that badger-to-cattle transmission events are amplified by onward transmission within local cattle populations. Hence, our findings inform debate even if they do not point to a single way forward.
References
- Krebs J, Anderson R, Clutton-Brock T, Morrison I, Young D, Donnelly C, Frost S and Woodroffe R. Bovine tuberculosis in cattle and badgers: Report to The Rt Hon Dr Jack Cunningham MP, Ministry of Agriculture, Fisheries and Food, 191pp, 1997.
Reference Link - Donnelly CA, Woodroffe R, Cox DR, Bourne FJ, Cheeseman CL, Clifton-Hadley RS, Wei G, Gettinby G, Gilks P, Jenkins H, Johnston WT, Le Fevre AM, McInerney JP, Morrison WI. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature. 2006 Feb 16;439(7078):843-6. PubMed PMID:16357869.
- Bourne J, Donnelly C, Cox D, Gettinby G, McInerney J, Morrison I, Woodroffe R. Bovine TB: the Scientific Evidence. A Science Base for a Sustainable Policy to Control TB in Cattle. Final Report of the Independent Scientific Group on Cattle TB presented to the Secretary of State for Environment, Food and Rural Affairs the Rt Hon David Miliband MP. 287pp. 2007.
Reference Link - Donnelly CA, Wei G, Johnston WT, Cox DR, Woodroffe R, Bourne FJ, Cheeseman CL, Clifton-Hadley RS, Gettinby G, Gilks P, Jenkins HE, Le Fevre AM, McInerney JP, Morrison WI. Impacts of widespread badger culling on cattle tuberculosis: concluding analyses from a large-scale field trial. Int J Infect Dis. 2007 Jul;11(4):300-8. PubMed PMID:17566777.
- Jenkins HE, Woodroffe R, Donnelly CA. The effects of annual widespread badger culls on cattle tuberculosis following the cessation of culling. Int J Infect Dis. 2008 Sep;12(5):457-65. PubMed PMID:18502675.
- Jenkins HE, Woodroffe R, Donnelly CA. The duration of the effects of repeated widespread badger culling on cattle tuberculosis following the cessation of culling. PLoS One. 2010 Feb 10;5(2):e9090. PubMed PMID:20161769.
- Independent Expert Panel. Oversees the design and analysis of the data collection on the humaneness, effectiveness (in terms of badger removal) and safety of the two badger culling pilots. 2013.
Reference Link - Welsh Government. IAA (Intensive Action Area) Badger Vaccination Report - Year 1. 2013.
Reference Link - Gates MC, Volkova VV, Woolhouse ME. Impact of changes in cattle movement regulations on the risks of bovine tuberculosis for Scottish farms. Prev Vet Med. 2013 Feb 1;108(2-3):125-36. PubMed PMID:22897858.
- Donnelly CA, Woodroffe R, Cox DR, Bourne J, Gettinby G, Le Fevre AM, McInerney JP, Morrison WI. Impact of localized badger culling on tuberculosis incidence in British cattle. Nature. 2003 Dec 18;426(6968):834-7. PubMed PMID:14634671.
- Vial F, Donnelly CA. Localized reactive badger culling increases risk of bovine tuberculosis in nearby cattle herds. Biol Lett. 2012 Feb 23;8(1):50-3. PubMed PMID:21752812.
- Donnelly CA, Hone J. 2010. Is there an association between levels of bovine tuberculosis in cattle herds and badgers? Stat Commun Infect Dis 2 (1): article 3. doi:10.2202/1948-4690.1000
Reference Link - Donnelly CA. Letter to Defra regarding an estimate of the proportion of cattle TB due to badgers. 2012.
Reference Link - Woodroffe R, Donnelly CA, Johnston WT, Bourne FJ, Cheeseman CL, Clifton-Hadley RS, et al. 2005. Spatial association of Mycobacterium bovis infection in cattle and badgers (Meles meles). J Appl Ecol 42:852-62. doi: 10.1111/j.1365-2664.2005.01081.x
- Barlow ND, Kean JM, Caldwell NP, Ryan TJ. Modelling the regional dynamics and management of bovine tuberculosis in New Zealand cattle herds. Prev Vet Med. 1998 Jul 17;36(1):25-38. PubMed PMID:9677625.
- Crawshaw TR, Griffiths IB, Clifton-Hadley RS. Comparison of a standard and a detailed postmortem protocol for detecting Mycobacterium bovis in badgers. Vet Rec. 2008 Oct 18;163(16):473-7. PubMed PMID:18931354.
- Woodroffe R, Donnelly CA, Jenkins HE, Johnston WT, Cox DR, Bourne FJ, Cheeseman CL, Delahay RJ, Clifton-Hadley RS, Gettinby G, Gilks P, Hewinson RG, McInerney JP, Morrison WI. Culling and cattle controls influence tuberculosis risk for badgers. Proc Natl Acad Sci U S A. 2006 Oct 3;103(40):14713-7. PubMed PMID:17015843.
- Robinson PA, Corner LA, Courcier EA, McNair J, Artois M, Menzies FD, Abernethy DA. BCG vaccination against tuberculosis in European badgers (Meles meles): a review. Comp Immunol Microbiol Infect Dis. 2012 Jul;35(4):277-87. PubMed PMID:22340983.
- Defra (Department for the Environment, Food and Rural Affairs). Bovine TB - Key conclusions from the meeting of scientific experts, held at Defra on 4th April 2011. 2011.
Reference Link - Chambers MA, Rogers F, Delahay RJ, Lesellier S, Ashford R, Dalley D, Gowtage S, Davé D, Palmer S, Brewer J, Crawshaw T, Clifton-Hadley R, Carter S, Cheeseman C, Hanks C, Murray A, Palphramand K, Pietravalle S, Smith GC, Tomlinson A, Walker NJ, Wilson GJ, Corner LA, Rushton SP, Shirley MD, Gettinby G, McDonald RA, Hewinson RG. Bacillus Calmette-Guérin vaccination reduces the severity and progression of tuberculosis in badgers. Proc Biol Sci. 2011 Jun 22;278(1713):1913-20. PubMed PMID:21123260.
- Carter SP, Chambers MA, Rushton SP, Shirley MD, Schuchert P, Pietravalle S, Murray A, Rogers F, Gettinby G, Smith GC, Delahay RJ, Hewinson RG, McDonald RA. BCG vaccination reduces risk of tuberculosis infection in vaccinated badgers and unvaccinated badger cubs. PLoS One. 2012;7(12):e49833. PubMed PMID:23251352.

Leave a Comment
You must be logged in to post a comment.